Soft robotic constrictor for in vitro modeling of dynamic tissue compression
- PMID: 34389738
- PMCID: PMC8363742
- DOI: 10.1038/s41598-021-94769-2
Soft robotic constrictor for in vitro modeling of dynamic tissue compression
Abstract
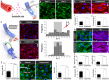
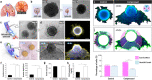
Here we present a microengineered soft-robotic in vitro platform developed by integrating a pneumatically regulated novel elastomeric actuator with primary culture of human cells. This system is capable of generating dynamic bending motion akin to the constriction of tubular organs that can exert controlled compressive forces on cultured living cells. Using this platform, we demonstrate cyclic compression of primary human endothelial cells, fibroblasts, and smooth muscle cells to show physiological changes in their morphology due to applied forces. Moreover, we present mechanically actuatable organotypic models to examine the effects of compressive forces on three-dimensional multicellular constructs designed to emulate complex tissues such as solid tumors and vascular networks. Our work provides a preliminary demonstration of how soft-robotics technology can be leveraged for in vitro modeling of complex physiological tissue microenvironment, and may enable the development of new research tools for mechanobiology and related areas.
© 2021. The Author(s).
Conflict of interest statement
D.H. is a co-founder of Vivodyne Inc. and holds equity in Vivodyne Inc. and Emulate Inc. J.P., J.S., E.B., Y.M., C.O., and V.S. declare no potential conflict of interest.
Figures



Similar articles
-
Design and Fabrication of a Three-Dimensional In Vitro System for Modeling Vascular Stenosis.Microsc Microanal. 2017 Aug;23(4):859-871. doi: 10.1017/S1431927617012302. Epub 2017 Jul 17. Microsc Microanal. 2017. PMID: 28712382
-
Microphysiological Engineering of Self-Assembled and Perfusable Microvascular Beds for the Production of Vascularized Three-Dimensional Human Microtissues.ACS Nano. 2019 Jul 23;13(7):7627-7643. doi: 10.1021/acsnano.9b00686. Epub 2019 Jun 18. ACS Nano. 2019. PMID: 31194909
-
Perfusion bioreactor for small diameter tissue-engineered arteries.Tissue Eng. 2004 May-Jun;10(5-6):930-41. doi: 10.1089/1076327041348536. Tissue Eng. 2004. PMID: 15265311
-
Construction of three-dimensional vascularized cardiac tissue with cell sheet engineering.J Control Release. 2015 May 10;205:83-8. doi: 10.1016/j.jconrel.2014.12.016. Epub 2014 Dec 16. J Control Release. 2015. PMID: 25523520 Review.
-
Roles of hemodynamic forces in vascular cell differentiation.Ann Biomed Eng. 2005 Jun;33(6):772-9. doi: 10.1007/s10439-005-3310-9. Ann Biomed Eng. 2005. PMID: 16078617 Review.
Cited by
-
Multicellular aligned bands disrupt global collective cell behavior.Acta Biomater. 2023 Jun;163:117-130. doi: 10.1016/j.actbio.2022.10.041. Epub 2022 Oct 26. Acta Biomater. 2023. PMID: 36306982 Free PMC article.
-
Simultaneous One-Pot Interpenetrating Network Formation to Expand 3D Processing Capabilities.Adv Mater. 2022 Jul;34(28):e2202261. doi: 10.1002/adma.202202261. Epub 2022 Jun 4. Adv Mater. 2022. PMID: 35510317 Free PMC article.
-
Soft bioreactor systems: a necessary step toward engineered MSK soft tissue?Front Robot AI. 2024 Apr 22;11:1287446. doi: 10.3389/frobt.2024.1287446. eCollection 2024. Front Robot AI. 2024. PMID: 38711813 Free PMC article. Review.
-
Development of a Pneumatic-Driven Fiber-Shaped Robot Scaffold for Use as a Complex 3D Dynamic Culture System.Biomimetics (Basel). 2023 Apr 21;8(2):170. doi: 10.3390/biomimetics8020170. Biomimetics (Basel). 2023. PMID: 37092422 Free PMC article.
-
Skeletal Muscle Tissue Engineering: From Tissue Regeneration to Biorobotics.Cyborg Bionic Syst. 2025 May 15;6:0279. doi: 10.34133/cbsystems.0279. eCollection 2025. Cyborg Bionic Syst. 2025. PMID: 40376483 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

