miR-141 mediates recovery from acute kidney injury
- PMID: 34389752
- PMCID: PMC8363617
- DOI: 10.1038/s41598-021-94984-x
miR-141 mediates recovery from acute kidney injury
Abstract
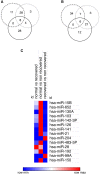
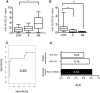
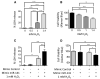
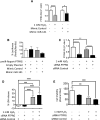
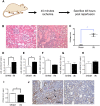
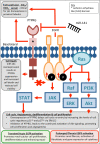
Acute kidney injury (AKI) is a global clinical problem characterised by a sudden decline in renal function and mortality as high as 60%. Current AKI biomarkers have limited ability to classify disease progression and identify underlying pathological mechanisms. Here we hypothesised that alterations in urinary microRNA profiles could predict AKI recovery/nonrecovery after 90 days, and that injury-specific changes would signify microRNA mediators of AKI pathology. Comparison of urinary microRNA profiles from AKI patients with controls detected significant injury-specific increases in miR-21, miR-126 and miR-141 (p < 0.05) and decreases in miR-192 (p < 0.001) and miR-204 (p < 0.05). Expression of miR-141 increased in renal proximal tubular epithelial cells (PTECs) under oxidative stress in vitro and unilateral ischaemic reperfusion injury in vivo. Forced miR-141 expression in the presence of H2O2 increased PTEC death and decreased cell viability. Of nine messenger RNA targets with two or more miR-141 3'-untranslated region binding sites, we confirmed protein tyrosine phosphatase receptor type G (PTPRG) as a direct miR-141 target in PTECs. PTPRG-specific siRNA knockdown under oxidative stress increased PTEC death and decreased cell viability. In conclusion, we detected significant alterations in five urinary microRNAs following AKI, and identified proximal tubular cell PTPRG as a putative novel therapeutic target.
© 2021. The Author(s).
Conflict of interest statement
T.B. and D.F. are inventors for patent WO/2017/129977 Chronic Kidney Disease Diagnostic. All other authors have no competing/conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

