A highly-sensitive genetically encoded temperature indicator exploiting a temperature-responsive elastin-like polypeptide
- PMID: 34389773
- PMCID: PMC8363741
- DOI: 10.1038/s41598-021-96049-5
A highly-sensitive genetically encoded temperature indicator exploiting a temperature-responsive elastin-like polypeptide
Abstract
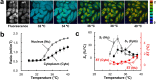
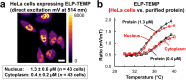
Genetically encoded temperature indicators (GETIs) allow for real-time measurement of subcellular temperature dynamics in live cells. However, GETIs have suffered from poor temperature sensitivity, which may not be sufficient to resolve small heat production from a biological process. Here, we develop a highly-sensitive GETI, denoted as ELP-TEMP, comprised of a temperature-responsive elastin-like polypeptide (ELP) fused with a cyan fluorescent protein (FP), mTurquoise2 (mT), and a yellow FP, mVenus (mV), as the donor and acceptor, respectively, of Förster resonance energy transfer (FRET). At elevated temperatures, the ELP moiety in ELP-TEMP undergoes a phase transition leading to an increase in the FRET efficiency. In HeLa cells, ELP-TEMP responded to the temperature from 33 to 40 °C with a maximum temperature sensitivity of 45.1 ± 8.1%/°C, which was the highest ever temperature sensitivity among hitherto-developed fluorescent nanothermometers. Although ELP-TEMP showed sensitivity not only to temperature but also to macromolecular crowding and self-concentration, we were able to correct the output of ELP-TEMP to achieve accurate temperature measurements at a subcellular resolution. We successfully applied ELP-TEMP to accurately measure temperature changes in cells induced by a local heat spot, even if the temperature difference was as small as < 1 °C, and to visualize heat production from stimulated Ca2+ influx in live HeLa cells induced by a chemical stimulation. Furthermore, we investigated temperatures in the nucleus and cytoplasm of live HeLa cells and found that their temperatures were almost the same within the temperature resolution of our measurement. Our study would contribute to better understanding of cellular temperature dynamics, and ELP-TEMP would be a useful GETI for the investigation of cell thermobiology.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

