Effect of adding hydrochlorothiazide to usual treatment of patients with acute decompensated heart failure: a randomized clinical trial
- PMID: 34389780
- PMCID: PMC8363660
- DOI: 10.1038/s41598-021-96002-6
Effect of adding hydrochlorothiazide to usual treatment of patients with acute decompensated heart failure: a randomized clinical trial
Erratum in
-
Author Correction: Effect of adding hydrochlorothiazide to usual treatment of patients with acute decompensated heart failure: a randomized clinical trial.Sci Rep. 2021 Aug 24;11(1):17370. doi: 10.1038/s41598-021-96943-y. Sci Rep. 2021. PMID: 34429503 Free PMC article. No abstract available.
Abstract
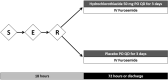
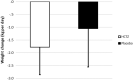
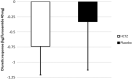
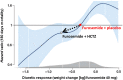
Acute decompensated heart failure (ADHF) is the leading cause of hospitalization in patients aged 65 years or older, and most of them present with congestion. The use of hydrochlorothiazide (HCTZ) may increase the response to loop diuretics. To evaluate the effect of adding HCTZ to furosemide on congestion and symptoms in patients with ADHF. This randomized clinical trial compared HCTZ 50 mg versus placebo for 3 days in patients with ADHF and signs of congestion. The primary outcome of the study was daily weight reduction. Secondary outcomes were change in creatinine, need for vasoactive drugs, change in natriuretic peptides, congestion score, dyspnea, thirst, and length of stay. Fifty-one patients were randomized-26 to the HCTZ group and 25 to the placebo group. There was an increment of 0.73 kg/day towards additional weight reduction in the HCTZ group (HCTZ: - 1.78 ± 1.08 kg/day vs placebo: - 1.05 ± 1.51 kg/day; p = 0.062). In post hoc analysis, the HCTZ group demonstrated significant weight reduction for every 40 mg of intravenous furosemide (HCTZ: - 0.74 ± 0.47 kg/40 mg vs placebo: - 0.33 ± 0.80 kg/40 mg; p = 0.032). There was a trend to increase in creatinine in the HCTZ group (HCTZ: 0.50 ± 0.37 vs placebo: 0.27 ± 0.40; p = 0.05) but no significant difference in onset of acute renal failure (HCTZ: 58% vs placebo: 41%; p = 0.38). No differences were found in the remaining outcomes. Adding hydrochlorothiazide to usual treatment of patients with acute decompensated heart failure did not cause significant difference in daily body weight reduction compared to placebo. In analysis adjusted to the dose of intravenous furosemide, adding HCTZ 50 mg to furosemide resulted in a significant synergistic effect on weight loss.Trial registration: The Brazilian Clinical Trials Registry (ReBEC), a publically accessible primary register that participates in the World Health Organization International Clinical Trial Registry Platform; number RBR-5qkn8h. Registered in 23/07/2019 (retrospectively), http://www.ensaiosclinicos.gov.br/rg/RBR-5qkn8h/ .
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

