Salt tolerance-based niche differentiation of soil ammonia oxidizers
- PMID: 34389794
- PMCID: PMC8776802
- DOI: 10.1038/s41396-021-01079-6
Salt tolerance-based niche differentiation of soil ammonia oxidizers
Abstract
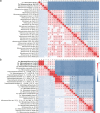
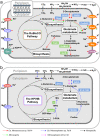
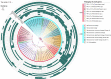
Ammonia oxidizers are key players in the global nitrogen cycle, yet little is known about their ecological performances and adaptation strategies for growth in saline terrestrial ecosystems. This study combined 13C-DNA stable-isotope probing (SIP) microcosms with amplicon and shotgun sequencing to reveal the composition and genomic adaptations of active ammonia oxidizers in a saline-sodic (solonetz) soil with high salinity and pH (20.9 cmolc exchangeable Na+ kg-1 soil and pH 9.64). Both ammonia-oxidizing archaea (AOA) and bacteria (AOB) exhibited strong nitrification activities, although AOB performed most of the ammonia oxidation observed in the solonetz soil and in the farmland soil converted from solonetz soil. Members of the Nitrosococcus, which are more often associated with aquatic habitats, were identified as the dominant ammonia oxidizers in the solonetz soil with the first direct labeling evidence, while members of the Nitrosospira were the dominant ammonia oxidizers in the farmland soil, which had much lower salinity and pH. Metagenomic analysis of "Candidatus Nitrosococcus sp. Sol14", a new species within the Nitrosococcus lineage, revealed multiple genomic adaptations predicted to facilitate osmotic and pH homeostasis in this extreme habitat, including direct Na+ extrusion/H+ import and the ability to increase intracellular osmotic pressure by accumulating compatible solutes. Comparative genomic analysis revealed that variation in salt-tolerance mechanisms was the primary driver for the niche differentiation of ammonia oxidizers in saline-sodic soils. These results demonstrate how ammonia oxidizers can adapt to saline-sodic soil with excessive Na+ content and provide new insights on the nitrogen cycle in extreme terrestrial ecosystems.
© 2021. The Author(s), under exclusive licence to International Society for Microbial Ecology.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Kuypers MMM, Marchant HK, Kartal B. The microbial nitrogen-cycling network. Nat Rev Microbiol. 2018;16:263–76. - PubMed
-
- Stein LY, Klotz MG. The nitrogen cycle. Curr Biol. 2016;26:R94–R98. - PubMed
-
- Erguder TH, Boon N, Wittebolle L, Marzorati M, Verstraete W. Environmental factors shaping the ecological niches of ammonia-oxidizing archaea. FEMS Microbiol Rev. 2009;33:855–69. - PubMed
-
- Nicol GW, Leininger S, Schleper C, Prosser JI. The influence of soil pH on the diversity, abundance and transcriptional activity of ammonia oxidizing archaea and bacteria. Environ Microbiol. 2008;10:2966–78. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

