Neuroimaging in Frontotemporal Dementia: Heterogeneity and Relationships with Underlying Neuropathology
- PMID: 34389969
- PMCID: PMC8423978
- DOI: 10.1007/s13311-021-01101-x
Neuroimaging in Frontotemporal Dementia: Heterogeneity and Relationships with Underlying Neuropathology
Abstract
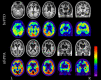
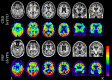
Frontotemporal dementia encompasses a group of clinical syndromes defined pathologically by degeneration of the frontal and temporal lobes. Historically, these syndromes have been challenging to diagnose, with an average of about three years between the time of symptom onset and the initial evaluation and diagnosis. Research in the field of neuroimaging has revealed numerous biomarkers of the various frontotemporal dementia syndromes, which has provided clinicians with a method of narrowing the differential diagnosis and improving diagnostic accuracy. As such, neuroimaging is considered a core investigative tool in the evaluation of neurodegenerative disorders. Furthermore, patterns of neurodegeneration correlate with the underlying neuropathological substrates of the frontotemporal dementia syndromes, which can aid clinicians in determining the underlying etiology and improve prognostication. This review explores the advancements in neuroimaging and discusses the phenotypic and pathologic features of behavioral variant frontotemporal dementia, semantic variant primary progressive aphasia, and nonfluent variant primary progressive aphasia, as seen on structural magnetic resonance imaging and positron emission tomography.
Keywords: Behavioral variant frontotemporal dementia; FTLD-TDP; FTLD-tau; Frontotemporal dementia; Frontotemporal lobar degeneration; Magnetic resonance imaging; Neuroimaging; Nonfluent agrammatic variant primary progressive aphasia; Positron emission tomography; Progressive nonfluent aphasia; Semantic dementia; Semantic variant primary progressive aphasia; TDP-43; Tau.
© 2021. The Author(s).
Figures




Similar articles
-
Imaging frontotemporal lobar degeneration.Curr Neurol Neurosci Rep. 2014 Oct;14(10):489. doi: 10.1007/s11910-014-0489-x. Curr Neurol Neurosci Rep. 2014. PMID: 25171901 Review.
-
TDP-43 subtypes are associated with distinct atrophy patterns in frontotemporal dementia.Neurology. 2010 Dec 14;75(24):2204-11. doi: 10.1212/WNL.0b013e318202038c. Neurology. 2010. PMID: 21172843 Free PMC article.
-
Atypical parkinsonian syndromes: a general neurologist's perspective.Eur J Neurol. 2018 Jan;25(1):41-58. doi: 10.1111/ene.13412. Epub 2017 Sep 28. Eur J Neurol. 2018. PMID: 28803444 Free PMC article. Review.
-
FTD spectrum: Neuroimaging across the FTD spectrum.Prog Mol Biol Transl Sci. 2019;165:187-223. doi: 10.1016/bs.pmbts.2019.05.009. Epub 2019 Jun 18. Prog Mol Biol Transl Sci. 2019. PMID: 31481163 Free PMC article. Review.
-
Primary progressive aphasia and the FTD-MND spectrum disorders: clinical, pathological, and neuroimaging correlates.Amyotroph Lateral Scler Frontotemporal Degener. 2019 May;20(3-4):146-158. doi: 10.1080/21678421.2018.1556695. Epub 2019 Jan 22. Amyotroph Lateral Scler Frontotemporal Degener. 2019. PMID: 30668155 Free PMC article.
Cited by
-
Frontotemporal Dementia and Late-Onset Bipolar Disorder: The Many Directions of a Busy Road.Front Psychiatry. 2021 Dec 2;12:768722. doi: 10.3389/fpsyt.2021.768722. eCollection 2021. Front Psychiatry. 2021. PMID: 34925096 Free PMC article. Review.
-
Dysphagia in primary progressive aphasia: Clinical predictors and neuroanatomical basis.Eur J Neurol. 2024 Sep;31(9):e16370. doi: 10.1111/ene.16370. Epub 2024 Jun 21. Eur J Neurol. 2024. PMID: 39012305 Free PMC article.
-
Research trends and hotspots for frontotemporal dementia from 2000 to 2022: a bibliometric analysis.Front Neurol. 2024 Jul 17;15:1399600. doi: 10.3389/fneur.2024.1399600. eCollection 2024. Front Neurol. 2024. PMID: 39087008 Free PMC article.
-
The Association Between Neurocognitive Disorders and Gustatory Dysfunction: A Systematic Review and Meta-Analysis.Neuropsychol Rev. 2024 Mar;34(1):192-213. doi: 10.1007/s11065-023-09578-3. Epub 2023 Feb 20. Neuropsychol Rev. 2024. PMID: 36806051 Free PMC article.
-
Single-cell RNA-seq reveals alterations in peripheral CX3CR1 and nonclassical monocytes in familial tauopathy.Genome Med. 2023 Jul 18;15(1):53. doi: 10.1186/s13073-023-01205-3. Genome Med. 2023. PMID: 37464408 Free PMC article.
References
-
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

