Scout accelerated motion estimation and reduction (SAMER)
- PMID: 34390505
- PMCID: PMC8616778
- DOI: 10.1002/mrm.28971
Scout accelerated motion estimation and reduction (SAMER)
Abstract
Purpose: To demonstrate a navigator/tracking-free retrospective motion estimation technique that facilitates clinically acceptable reconstruction time.
Methods: Scout accelerated motion estimation and reduction (SAMER) uses a single 3-5 s, low-resolution scout scan and a novel sequence reordering to independently determine motion states by minimizing the data-consistency error in a SENSE plus motion forward model. This eliminates time-consuming alternating optimization as no updates to the imaging volume are required during the motion estimation. The SAMER approach was assessed quantitatively through extensive simulation and was evaluated in vivo across multiple motion scenarios and clinical imaging contrasts. Finally, SAMER was synergistically combined with advanced encoding (Wave-CAIPI) to facilitate rapid motion-free imaging.
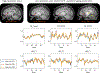
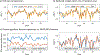
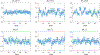
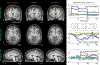
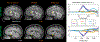
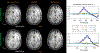
Results: The highly accelerated scout provided sufficient information to achieve accurate motion trajectory estimation (accuracy ~0.2 mm or degrees). The novel sequence reordering improved the stability of the motion parameter estimation and image reconstruction while preserving the clinical imaging contrast. Clinically acceptable computation times for the motion estimation (~4 s/shot) are demonstrated through a fully separable (non-alternating) motion search across the shots. Substantial artifact reduction was demonstrated in vivo as well as corresponding improvement in the quantitative error metric. Finally, the extension of SAMER to Wave-encoding enabled rapid high-quality imaging at up to R = 9-fold acceleration.
Conclusion: SAMER significantly improved the computational scalability for retrospective motion estimation and correction.
Keywords: parallel imaging; retrospective motion correction; wave-CAIPI.
© 2021 International Society for Magnetic Resonance in Medicine.
Figures
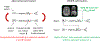


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

