Safety, tolerability, pharmacokinetics and pharmacodynamics of single oral doses of BI 187004, an inhibitor of 11beta-hydroxysteroid dehydrogenase-1, in healthy male volunteers with overweight or obesity
- PMID: 34391480
- PMCID: PMC8364686
- DOI: 10.1186/s40842-021-00130-x
Safety, tolerability, pharmacokinetics and pharmacodynamics of single oral doses of BI 187004, an inhibitor of 11beta-hydroxysteroid dehydrogenase-1, in healthy male volunteers with overweight or obesity
Abstract
Background: The study characterizes safety, tolerability, pharmacokinetic and pharmacodynamic profiles of single rising doses of the 11beta-hydroxysteroid dehydrogenase-1 (11beta-HSD1) inhibitor BI 187004 in healthy men with overweight or obesity.
Methods: This was a randomized, double-blind, parallel group, placebo-controlled study with administration of 2.5-360 mg BI 187004 or placebo once daily as single dose in 72 healthy male volunteers with overweight or obesity. Assessments included 11beta-HSD1 inhibition in the liver (assessed indirectly by urinary tetrahydrocortisol/tetrahydrocortisone ratio) and in subcutaneous adipose tissue ex vivo and determination of hypothalamus-pituitary-adrenal axis hormones.
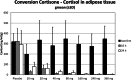
Results: BI 187004 was well tolerated and safe in all tested dose groups. The incidence of drug-related adverse events was 16.7% (n = 9) for all 9 BI 187004 dose groups and 5.9% (n = 1) for placebo. All treatment groups were similar concerning kind and intensity of adverse events. No clinically relevant deviations in clinical laboratory or ECG parameters were reported. Exposure of BI 187004 increased non-proportionally over the entire dose range tested. The geometric mean apparent terminal half-life decreased from 33.5 h (5 mg) to 14.5 h (160 mg) remaining stable up to 360 mg. Renal excretion of BI 187004 was low (3-5%). Urinary tetrahydrocortisol/tetrahydrocortisone ratio decreased, indicating liver 11beta-HSD1 inhibition. Median inhibition of 11beta-HSD1 in subcutaneous adipose tissue biopsies following single dosing ranged from 86.8% (10 mg) to 99.5% (360 mg) after 10 h and from 59.4% (10 mg) to 98.6% (360 mg) after 24 h.
Conclusions: BI 187004 as single dose was safe and well tolerated and is suitable for once daily dosing. There was significant, sustained 11beta-HSD1 inhibition in liver and adipose tissue.
Trial registration: ClinicalTrials.gov, NCT01587417 , registered on 26-Apr-2012.
Keywords: 11beta-Hydroxysteroid dehydrogynase-1 inhibitor; BI 187004; Pharmacodynamics; Pharmacokinetics; Single rising dose; Type 2 diabetes.
© 2021. The Author(s).
Conflict of interest statement
The institution of TH received research grants from the following pharmaceutical companies: Adocia, Aerami Pharmaceutics, Becton Dickinson, Biocon, Boehringer Ingelheim, Eli Lilly, Gan Lee Pharmaceuticals, MedImmune, Merck, Mylan, Nordic Bioscience, Novo Nordisk, Poxel, Sanofi-Aventis, Xeris, and Zealand Pharma in the past 12 months. In addition, TH received travel grants, consulting fees, and speaker honoraria from Eli Lilly, Mylan and Novo Nordisk.
SB, AJ, CSche, CSchoe, and UGM are employees of Boehringer Ingelheim.
Figures


References
-
- Sjostrand M, Jansson PA, Palming J, School D, Gill D, Rees A, Sjogren L, Persson T, Eriksson JW. Repeated measurements of 11beta-HSD-1 activity in subcutaneous adipose tissue from lean, abdominally obese, and type 2 diabetes subjects–no change following a mixed meal. Horm Metab Res. 2010;42:798–802. doi: 10.1055/s-0030-1254134. - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

