Phosphorylation of slit diaphragm proteins NEPHRIN and NEPH1 upon binding of HGF promotes podocyte repair
- PMID: 34391780
- PMCID: PMC8429977
- DOI: 10.1016/j.jbc.2021.101079
Phosphorylation of slit diaphragm proteins NEPHRIN and NEPH1 upon binding of HGF promotes podocyte repair
Abstract
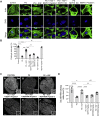
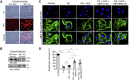
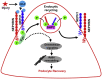
Phosphorylation (activation) and dephosphorylation (deactivation) of the slit diaphragm proteins NEPHRIN and NEPH1 are critical for maintaining the kidney epithelial podocyte actin cytoskeleton and, therefore, proper glomerular filtration. However, the mechanisms underlying these events remain largely unknown. Here we show that NEPHRIN and NEPH1 are novel receptor proteins for hepatocyte growth factor (HGF) and can be phosphorylated independently of the mesenchymal epithelial transition receptor in a ligand-dependent fashion through engagement of their extracellular domains by HGF. Furthermore, we demonstrate SH2 domain-containing protein tyrosine phosphatase-2-dependent dephosphorylation of these proteins. To establish HGF as a ligand, purified baculovirus-expressed NEPHRIN and NEPH1 recombinant proteins were used in surface plasma resonance binding experiments. We report high-affinity interactions of NEPHRIN and NEPH1 with HGF, although NEPHRIN binding was 20-fold higher than that of NEPH1. In addition, using molecular modeling we constructed peptides that were used to map specific HGF-binding regions in the extracellular domains of NEPHRIN and NEPH1. Finally, using an in vitro model of cultured podocytes and an ex vivo model of Drosophila nephrocytes, as well as chemically induced injury models, we demonstrated that HGF-induced phosphorylation of NEPHRIN and NEPH1 is centrally involved in podocyte repair. Taken together, this is the first study demonstrating a receptor-based function for NEPHRIN and NEPH1. This has important biological and clinical implications for the repair of injured podocytes and the maintenance of podocyte integrity.
Keywords: HGF; SHP-2; phosphorylation–dephosphorylation; podocytes.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures






References
-
- Benzing T. Signaling at the slit diaphragm. J. Am. Soc. Nephrol. 2004;15:1382–1391. - PubMed
-
- Patrakka J., Tryggvason K. Nephrin--a unique structural and signaling protein of the kidney filter. Trends Mol. Med. 2007;13:396–403. - PubMed
-
- Donoviel D.B., Freed D.D., Vogel H., Potter D.G., Hawkins E., Barrish J.P., Mathur B.N., Turner C.A., Geske R., Montgomery C.A., Starbuck M., Brandt M., Gupta A., Ramirez-Solis R., Zambrowicz B.P. Proteinuria and perinatal lethality in mice lacking NEPH1, a novel protein with homology to NEPHRIN. Mol. Cell. Biol. 2001;21:4829–4836. - PMC - PubMed
-
- Rantanen M., Palmén T., Pätäri A., Ahola H., Lehtonen S., Aström E., Floss T., Vauti F., Wurst W., Ruiz P., Kerjaschki D., Holthöfer H. Nephrin TRAP mice lack slit diaphragms and show fibrotic glomeruli and cystic tubular lesions. J. Am. Soc. Nephrol. 2002;13:1586–1594. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

