Sacubitril-valsartan as a treatment for apparent resistant hypertension in patients with heart failure and preserved ejection fraction
- PMID: 34392331
- PMCID: PMC8455346
- DOI: 10.1093/eurheartj/ehab499
Sacubitril-valsartan as a treatment for apparent resistant hypertension in patients with heart failure and preserved ejection fraction
Abstract
Aims: Patients with heart failure and preserved ejection fraction (HFpEF) frequently have difficult-to-control hypertension. We examined the effect of neprilysin inhibition on 'apparent resistant hypertension' in patients with HFpEF in the PARAGON-HF trial, which compared the effect of sacubitril-valsartan with valsartan.
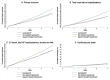
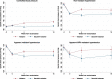
Methods and results: In this post hoc analysis, patients were categorized according to systolic blood pressure at the end of the valsartan run-in (n = 4795). 'Apparent resistant hypertension' was defined as systolic blood pressure ≥140 mmHg (≥135 mmHg if diabetes) despite treatment with valsartan, a calcium channel blocker, and a diuretic. 'Apparent mineralocorticoid receptor antagonist (MRA)-resistant' hypertension was defined as systolic blood pressure ≥140 mmHg (≥135 mmHg if diabetes) despite the above treatments and an MRA. The primary outcome in the PARAGON-HF trial was a composite of total hospitalizations for heart failure and death from cardiovascular causes. We examined clinical endpoints and the safety of sacubitril-valsartan according to the hypertension category. We also examined reductions in blood pressure from the end of valsartan run-in to Weeks 4 and 16 after randomization. Overall, 731 patients (15.2%) had apparent resistant hypertension and 135 (2.8%) had apparent MRA-resistant hypertension. The rate of the primary outcome was higher in patients with apparent resistant hypertension [17.3; 95% confidence interval (CI) 15.6-19.1 per 100 person-years] compared to those with a controlled systolic blood pressure (13.4; 12.7-14.3 per 100 person-years), with an adjusted rate ratio of 1.28 (95% CI 1.05-1.57). The reduction in systolic blood pressure at Weeks 4 and 16, respectively, was greater with sacubitril-valsartan vs. valsartan in patients with apparent resistant hypertension [-4.8 (-7.0 to -2.5) and 3.9 (-6.6 to -1.3) mmHg] and apparent MRA-resistant hypertension [-8.8 (-14.0 to -3.5) and -6.3 (-12.5 to -0.1) mmHg]. The proportion of patients with apparent resistant hypertension achieving a controlled systolic blood pressure by Week 16 was 47.9% in the sacubitril-valsartan group and 34.3% in the valsartan group [adjusted odds ratio (OR) 1.78, 95% CI 1.30-2.43]. In patients with apparent MRA-resistant hypertension, the respective proportions were 43.6% vs. 28.4% (adjusted OR 2.63, 95% CI 1.18-5.89).
Conclusion: Sacubitril-valsartan may be useful in treating apparent resistant hypertension in patients with HFpEF, even in those who continue to have an elevated blood pressure despite treatment with at least four antihypertensive drug classes, including an MRA.
Clinical trial registration: PARAGON-HF: ClinicalTrials.gov Identifier NCT01920711.
Keywords: Blood pressure; Heart failure; Preserved ejection fraction; Sacubitril–valsartan.
© The Author(s) 2021. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures

Comment in
-
Sacubitril/valsartan for heart failure with preserved ejection fraction and resistant hypertension: one shot for a double strike?Eur Heart J. 2021 Sep 21;42(36):3753-3755. doi: 10.1093/eurheartj/ehab489. Eur Heart J. 2021. PMID: 34392358 No abstract available.
References
-
- Slivnick J, Lampert BC. Hypertension and heart failure. Heart Fail Clin 2019;15:531–541. - PubMed
-
- Gladden JD, Chaanine AH, Redfield MM. Heart failure with preserved ejection fraction. Annu Rev Med 2018;69:65–79. - PubMed
-
- Tadic M, Cuspidi C, Frydas A, Grassi G. The role of arterial hypertension in development heart failure with preserved ejection fraction: just a risk factor or something more? Heart Fail Rev 2018;23:631–639. - PubMed
-
- Gupta T, Rezan T, Krim SR. Managing hypertension in patients with heart failure. Curr Opin Cardiol 2019;34:359–366. - PubMed
-
- Shah SJ, Gheorghiade M. Heart failure with preserved ejection fraction: treat now by treating comorbidities. JAMA 2008;300:431–433. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

