Prolonged estrogen deprivation triggers a broad immunosuppressive phenotype in breast cancer cells
- PMID: 34392603
- PMCID: PMC8732350
- DOI: 10.1002/1878-0261.13083
Prolonged estrogen deprivation triggers a broad immunosuppressive phenotype in breast cancer cells
Abstract
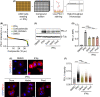
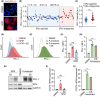
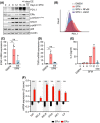
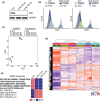
Among others, expression levels of programmed cell death 1 ligand 1 (PD-L1) have been explored as biomarkers of the response to immune checkpoint inhibitors in cancer therapy. Here, we present the results of a chemical screen that interrogated how medically approved drugs influence PD-L1 expression. As expected, corticosteroids and inhibitors of Janus kinases were among the top PD-L1 downregulators. In addition, we identified that PD-L1 expression is induced by antiestrogenic compounds. Transcriptomic analyses indicate that chronic estrogen receptor alpha (ERα) inhibition triggers a broad immunosuppressive program in ER-positive breast cancer cells, which is subsequent to their growth arrest and involves the activation of multiple immune checkpoints together with the silencing of the antigen-presenting machinery. Accordingly, estrogen-deprived MCF7 cells are resistant to T-cell-mediated cell killing, in a manner that is independent of PD-L1, but which is reverted by estradiol. Our study reveals that while antiestrogen therapies efficiently limit the growth of ER-positive breast cancer cells, they concomitantly trigger a transcriptional program that favors their immune evasion.
Keywords: HLA; PD-L1; breast cancer; estrogen receptor; immunotherapy; inflammation.
© 2021 The Authors. Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA & Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68, 394–424. - PubMed
-
- Prall OW, Rogan EM & Sutherland RL (1998) Estrogen regulation of cell cycle progression in breast cancer cells. J Steroid Biochem Mol Biol 65, 169–174. - PubMed
-
- Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, Dieras V, Hegg R, Im SA, Shaw Wright G et al. (2018) Atezolizumab and Nab‐paclitaxel in advanced triple‐negative breast cancer. N Engl J Med 379, 2108–2121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

