Visceral sensitivity modulation by faecal microbiota transplantation: the active role of gut bacteria in pain persistence
- PMID: 34393197
- PMCID: PMC9009324
- DOI: 10.1097/j.pain.0000000000002438
Visceral sensitivity modulation by faecal microbiota transplantation: the active role of gut bacteria in pain persistence
Abstract
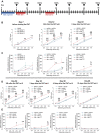
Recent findings linked gastrointestinal disorders characterized by abdominal pain to gut microbiota composition. The present work aimed to evaluate the power of gut microbiota as a visceral pain modulator and, consequently, the relevance of its manipulation as a therapeutic option in reversing postinflammatory visceral pain persistence. Colitis was induced in mice by intrarectally injecting 2,4-dinitrobenzenesulfonic acid (DNBS). The effect of faecal microbiota transplantation from viscerally hypersensitive DNBS-treated and naive donors was evaluated in control rats after an antibiotic-mediated microbiota depletion. Faecal microbiota transplantation from DNBS donors induced a long-lasting visceral hypersensitivity in control rats. Pain threshold trend correlated with major modifications in the composition of gut microbiota and short chain fatty acids. By contrast, no significant alterations of colon histology, permeability, and monoamines levels were detected. Finally, by manipulating the gut microbiota of DNBS-treated animals, a counteraction of persistent visceral pain was achieved. The present results provide novel insights into the relationship between intestinal microbiota and visceral hypersensitivity, highlighting the therapeutic potential of microbiota-targeted interventions.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the International Association for the Study of Pain.
Conflict of interest statement
The authors have declared that no conflicts of interest exists.
Figures






References
-
- Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 2018;23:716–24. - PubMed
-
- Antonioli L, Fornai M, Colucci R, Awwad O, Ghisu N, Tuccori M, Del Tacca M, Blandizzi C. Differential recruitment of high affinity A1 and A2A adenosine receptors in the control of colonic neuromuscular function in experimental colitis. Eur J Pharmacol 2011;650:639–49. - PubMed
-
- Antonioli L, Fornai M, Colucci R, Ghisu N, Da Settimo F, Natale G, Kastsiuchenka O, Duranti E, Virdis A, Vassalle C, La Motta C, Mugnaini L, Breschi MC, Blandizzi C, Del Taca M. Inhibition of adenosine deaminase attenuates inflammation in experimental colitis. J Pharmacol Exp Ther 2007;322:435–42. - PubMed
-
- Banasiewicz T, Krokowicz L, Stojcev Z, Kaczmarek BF, Kaczmarek E, Maik J, Marciniak R, Krokowicz P, Walkowiak J, Drews M. Microencapsulated sodium butyrate reduces the frequency of abdominal pain in patients with irritable bowel syndrome. Colorectal Dis 2013;15:204–9. - PubMed
-
- Bandyopadhyaya A, Rajagopalan DR, Rath NP, Herrold A, Rajagopalan R, Napier TC, Tedford CE, Rajagopalan P. The synthesis and receptor binding affinities of DDD-016, a novel, potential, atypical antipsychotic. Medchemcomm 2012;3:580–3.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

