Clinical Strains of Pseudomonas aeruginosa Secrete LasB Elastase to Induce Hemorrhagic Diffuse Alveolar Damage in Mice
- PMID: 34393497
- PMCID: PMC8354736
- DOI: 10.2147/JIR.S322960
Clinical Strains of Pseudomonas aeruginosa Secrete LasB Elastase to Induce Hemorrhagic Diffuse Alveolar Damage in Mice
Abstract
Background: Acute lung injury and acute respiratory distress syndrome (ALI/ARDS) are most often caused by bacterial pneumonia and characterized by severe dyspnea and high mortality. Knowledge about the lung injury effects of current clinical bacterial strains is lacking. The aim of this study was to investigate the ability of representative pathogenic bacteria isolated from patients to cause ALI/ARDS in mice and identify the major virulence factor.
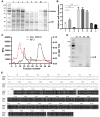
Methods: Seven major bacterial species were isolated from clinical sputum and unilaterally instilled into the mouse airway. A histology study was performed to determine the lung injury effect. Virulence genes were examined by PCR. Sequence types of P. aeruginosa strains were identified by MLST. LC-MS/MS was used to analysis the bacterial exoproducts proteome. LasB was purified through a DEAE-cellulose column, and its toxicity was tested both in vitro and in vivo.
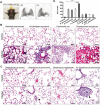
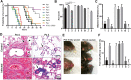
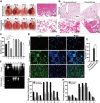
Results: Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus agalactiae, Acinetobacter baumannii, Klebsiella pneumoniae, Pseudomonas aeruginosa and Escherichia coli were randomly separated and tested 3 times. Among them, gram-negative bacteria have much more potential to cause acute lung injury than gram-positive bacteria. However, P. aeruginosa is the only pathogen that induces diffuse alveolar damage, hemorrhage and hyaline membranes in the lungs of mice. The lung injury effect is associated with the excreted LasB elastase. Purified LasB recapitulated lung injury similar to P. aeruginosa infection in vivo. We found that this was due to the powerful degradation effect of LasB on the extracellular matrix of the lung and key proteins in the coagulation cascade without inducing obvious cellular apoptosis. We also report for the first time that LasB could induce DIC-like coagulopathy in vitro.
Conclusion: P. aeruginosa strains are most capable of inducing ALI/ARDS in mice among major clinical pathogenic bacteria tested, and this ability is specifically attributed to their LasB production.
Keywords: ALI/ARDS; LasB elastase; Pseudomonas aeruginosa; unilateral lung injury.
© 2021 Zhu et al.
Conflict of interest statement
The authors declare no conflicts of interest in this work.
Figures
References
LinkOut - more resources
Full Text Sources

