Construction of transgenic Ipomoea obscura that exhibits new reddish leaf and flower colors due to introduction of β-carotene ketolase and hydroxylase genes
- PMID: 34393600
- PMCID: PMC8329268
- DOI: 10.5511/plantbiotechnology.21.0309a
Construction of transgenic Ipomoea obscura that exhibits new reddish leaf and flower colors due to introduction of β-carotene ketolase and hydroxylase genes
Abstract
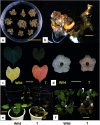
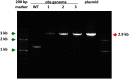
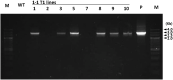
Ipomoea obscura, small white morning glory, is an ornamental plant belonging to the family Convolvulaceae, and cultivated worldwide. I. obscura generates white petals including a pale-yellow colored star-shaped center (flower vein). Its fully opened flowers were known to accumulate trace amounts of carotenoids such as β-carotene. In the present study, the embryogenic calli of I. obscura, were successfully produced through its immature embryo culture, and co-cultured with Agrobacterium tumefaciens carrying the β-carotene 4,4'-ketolase (crtW) and β-carotene 3,3'-hydroxylase (crtZ) genes for astaxanthin biosynthesis in addition to the isopentenyl diphosphate isomerase (idi) and hygromycin resistance genes. Transgenic plants, in which these four genes were introduced, were regenerated from the infected calli. They generated bronze (reddish green) leaves and novel petals that exhibited a color change from pale-yellow to pale-orange in the star-shaped center part. Especially, the color of their withered leaves changed drastically. HPLC-PDA-MS analysis showed that the expanded leaves of a transgenic line (T0) produced astaxanthin (5.2% of total carotenoids), adonirubin (3.9%), canthaxanthin (3.8%), and 3-hydroxyechinenone (3.6%), which indicated that these ketocarotenoids corresponded to 16.5% of the total carotenoids produced there (530 µg g-1 fresh weight). Furthermore, the altered traits of the transgenic plants were found to be inherited to their progenies by self-crossing.
Keywords: Ipomoea obscura; astaxanthin; embryogenic callus; transgenic plant.
© 2021 Japanese Society for Plant Biotechnology.
Conflict of interest statement
Disclosure of potential conflicts of interestThe authors declare that they have no conflicts of interest.
Figures



References
-
- Fujisawa M, Takita E, Harada H, Sakurai N, Suzuki H, Ohyama K, Shibata D, Misawa N (2009) Pathway engineering of Brassica napus seeds using multiple key-enzyme genes involved in ketocarotenoid formation. J Exp Bot 60: 1319–1332 - PubMed
-
- Harada H, Maoka T, Osawa A, Hattan J, Kanamoto H, Shindo K, Otomatsu T, Misawa N (2014) Construction of transplastomic lettuce (Lactuca sativa) dominantly producing astaxanthin fatty acid esters and detailed chemical analysis of generated carotenoids. Transgenic Res 23: 303–315 - PubMed
-
- Hasunuma T, Miyazawa SI, Yoshimura S, Shinzaki Y, Tomizawa KI, Shindo K, Choi SK, Misawa N, Miyake C (2008) Biosynthesis of astaxanthin in tobacco leaves by transplastomic engineering. Plant J 55: 857–868 - PubMed
-
- Hirschberg J (2001) Carotenoid biosynthesis in flowering plants. Curr Opin Plant Biol 4: 210–218 - PubMed
-
- Hoshino A, Mizuno T, Shimizu K, Mori S, Fukada-Tanaka S, Furukawa K, Ishiguro K, Tanaka Y, Iida S (2019) Generation of yellow flowers of the Japanese morning glory by engineering its flavonoid biosynthetic pathway toward aurones. Plant Cell Physiol 60: 1871–1879 - PubMed
LinkOut - more resources
Full Text Sources
