Vaccine Induced Immune Thrombotic Thrombocytopenia Causing a Severe Form of Cerebral Venous Thrombosis With High Fatality Rate: A Case Series
- PMID: 34393988
- PMCID: PMC8363077
- DOI: 10.3389/fneur.2021.721146
Vaccine Induced Immune Thrombotic Thrombocytopenia Causing a Severe Form of Cerebral Venous Thrombosis With High Fatality Rate: A Case Series
Abstract
During a 2-week period, we have encountered five cases presenting with the combination of cerebral venous thrombosis (CVT), intracerebral hemorrhage and thrombocytopenia. A clinical hallmark was the rapid and severe progression of disease in spite of maximum treatment efforts, resulting in fatal outcome in for 4 out of 5 patients. All cases had received ChAdOx1 nCov-19 vaccine 1-2 weeks earlier and developed a characteristic syndrome thereafter. The rapid progressive clinical course and high fatality rate of CVT in combination with thrombocytopenia in such a cluster and in otherwise healthy adults is a recent phenomenon. Cerebral autopsy findings were those of venous hemorrhagic infarctions and thrombi in dural venous sinuses, including thrombus material apparently rich in thrombocytes, leukocytes and fibrin. Vessel walls were free of inflammation. Extra-cerebral manifestations included leech-like thrombi in large veins, fibrin clots in small venules and scattered hemorrhages on skin and membranes. CVT with thrombocytopenia after adenovirus vectored COVID-19 vaccination is a new clinical syndrome that needs to be recognized by clinicians, is challenging to treat and seems associated with a high mortality rate.
Keywords: COVID-19; COVID-19 vaccine AstraZeneca; ChAdOx1 nCoV-19; SARS-CoV-2 virus; cerebral venous thrombosis; complication; sinus vein thrombosis; thrombocytopenia.
Copyright © 2021 Wiedmann, Skattør, Stray-Pedersen, Romundstad, Antal, Marthinsen, Sørvoll, Leiknes Ernstsen, Lund, Holme, Johansen, Brunborg, Aamodt, Schultz, Skagen and Skjelland.
Conflict of interest statement
MW research grants from the South-Eastern Norway Regional Health Authority (grant number 2014060), ownership of stock Biontech/Pfizer. IS reports that her spouse is the CFO in ArcticZymes Technologies. CL personal fees from Bristol Myers Squibb. PH personal fees from Takeda, grants and personal fees from SOBI, grants and personal fees from Bayer, grants and personal fees from Pfizer, personal fees from Roche, personal fees from Octapharma, personal fees from NovoNordisk, personal fees from CSL, personal fees from BMS. AA personal fees from Bayer, personal fees from Boehringer Ingelheim, personal fees from Roche, personal fees from Allergan, personal fees from Novartis, personal fees from Teva. NS personal fees from BMS/Pfizer, personal fees from Bayer. KS personal fees from Bayer. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
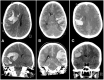
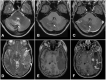
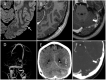
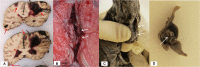
References
-
- Norwegian Institute of Public Health . COVID 19 Ukerapport — uke 15 A. (2021). Available online at: https://www.fhi.no/contentassets/8a971e7b0a3c4a06bdbf381ab52e6157/vedleg... (accessed April 27, 2021).
-
- Saposnik G, Barinagarrementeria F, Brown RD, Jr, Bushnell CD, Cucchiara B, Cushman M, et al. . Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American heart association/American stroke association. Stroke. (2011) 42:1158–92. 10.1161/STR.0b013e31820a8364 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

