Pathogenetic Interplay Between IL-6 and Tryptophan Metabolism in an Experimental Model of Obesity
- PMID: 34394118
- PMCID: PMC8361489
- DOI: 10.3389/fimmu.2021.713989
Pathogenetic Interplay Between IL-6 and Tryptophan Metabolism in an Experimental Model of Obesity
Abstract
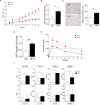
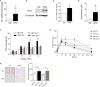
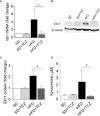
Obesity is a metabolic disease characterized by a state of chronic, low-grade inflammation and dominated by pro-inflammatory cytokines such as IL-6. Indoleamine 2,3-dioxygenase 1 (IDO1) is an enzyme that catalyzes the first step in the kynurenine pathway by transforming l-tryptophan (Trp) into l-kynurenine (Kyn), a metabolite endowed with anti-inflammatory and immunoregulatory effects. In dendritic cells, IL-6 induces IDO1 proteasomal degradation and shuts down IDO1-mediated immunosuppressive effects. In tumor cells, IL-6 upregulates IDO1 expression and favors tumor immune escape mechanisms. To investigate the role of IDO1 and its possible relationship with IL-6 in obesity, we induced the disease by feeding mice with a high fat diet (HFD). Mice on a standard diet were used as control. Experimental obesity was associated with high IDO1 expression and Kyn levels in the stromal vascular fraction of visceral white adipose tissue (SVF WAT). IDO1-deficient mice on HFD gained less weight and were less insulin resistant as compared to wild type counterparts. Administration of tocilizumab (TCZ), an IL-6 receptor (IL-6R) antagonist, to mice on HFD significantly reduced weight gain, controlled adipose tissue hypertrophy, increased insulin sensitivity, and induced a better glucose tolerance. TCZ also induced a dramatic inhibition of IDO1 expression and Kyn production in the SVF WAT. Thus our data indicated that the IL-6/IDO1 axis may play a pathogenetic role in a chronic, low-grade inflammation condition, and, perhaps most importantly, IL-6R blockade may be considered a valid option for obesity treatment.
Keywords: IL-6 receptor (IL-6R); experimental obesity; high fat diet (HFD); indoleamine 2, 3 dioxygenase 1 (IDO1); tocilizumab (TCZ); tryptophan metabolism; white adipose tissue (WAT).
Copyright © 2021 Mondanelli, Albini, Orecchini, Pallotta, Belladonna, Ricci, Grohmann and Orabona.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

