Lipid nanoparticles for mRNA delivery
- PMID: 34394960
- PMCID: PMC8353930
- DOI: 10.1038/s41578-021-00358-0
Lipid nanoparticles for mRNA delivery
Abstract
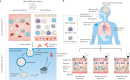
Messenger RNA (mRNA) has emerged as a new category of therapeutic agent to prevent and treat various diseases. To function in vivo, mRNA requires safe, effective and stable delivery systems that protect the nucleic acid from degradation and that allow cellular uptake and mRNA release. Lipid nanoparticles have successfully entered the clinic for the delivery of mRNA; in particular, lipid nanoparticle-mRNA vaccines are now in clinical use against coronavirus disease 2019 (COVID-19), which marks a milestone for mRNA therapeutics. In this Review, we discuss the design of lipid nanoparticles for mRNA delivery and examine physiological barriers and possible administration routes for lipid nanoparticle-mRNA systems. We then consider key points for the clinical translation of lipid nanoparticle-mRNA formulations, including good manufacturing practice, stability, storage and safety, and highlight preclinical and clinical studies of lipid nanoparticle-mRNA therapeutics for infectious diseases, cancer and genetic disorders. Finally, we give an outlook to future possibilities and remaining challenges for this promising technology.
Keywords: Drug delivery; Drug development.
© Springer Nature Limited 2021, corrected publication 2021.
Conflict of interest statement
Competing interestsY.D. is a scientific advisory board member of Oncorus, Inc. and serves as a consultant of Rubius Therapeutics. T.Z. is an employee of Moderna, Inc. R.L. is a founding scientific advisory board member of Alnylam and a founder and board member of Moderna, Inc. A list of entities with which R.L. is involved, compensated or uncompensated is provided in the supplementary information. X.H. declares no competing interests.
Figures



References
-
- Cobb M. Who discovered messenger RNA? Curr. Biol. 2015;25:R526–R532. - PubMed
-
- Sahin U, Karikó K, Türeci Ö. mRNA-based therapeutics — developing a new class of drugs. Nat. Rev. Drug Discov. 2014;13:759–780. - PubMed
-
- Wolff JA, et al. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. - PubMed
-
- Langer R, Folkman J. Polymers for the sustained release of proteins and other macromolecules. Nature. 1976;263:797–800. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
