Cross-validation of a high-performance liquid chromatography nevirapine plasma assay in a resource-limited setting in Zimbabwe
- PMID: 34395199
- PMCID: PMC8335789
- DOI: 10.4102/ajlm.v10i1.1264
Cross-validation of a high-performance liquid chromatography nevirapine plasma assay in a resource-limited setting in Zimbabwe
Abstract
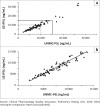
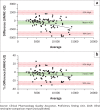
An international HIV pharmacology specialty laboratory (PSL) was established at the University of Zimbabwe to increase bioanalytical and investigator capacities. Quantitation of plasma nevirapine in samples from the AIDS Clinical Trials Group protocol 5279 was compared between the University of Nebraska Medical Center PSL and the University of Zimbabwe PSL. Both PSLs employed internally developed methods utilising reverse-phase high-performance liquid chromatography with ultraviolet detection. Eighty-seven percent of the cross-validation results exhibited ± 20% difference.
Keywords: HIV; cross-validation; high-performance liquid chromatography; root-cause analysis.
© 2021. The Authors.
Conflict of interest statement
The authors declare that they have no financial or personal relationships that may have inappropriately influenced them in writing this article.
Figures
References
-
- Bolaris MA, Keller MA, Robbins BL, Podany AT, Fletcher CV. Nevirapine plasma concentrations in human immunodeficiency virus-exposed neonates receiving high-dose nevirapine prophylaxis as part of 3-drug regimen. J Pediatric Infect Dis Soc. 2017. March 1;6(1):102–104. 10.1093/jpids/piv084 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
