Prognostic Role of Tumor Mutational Burden in Cancer Patients Treated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis
- PMID: 34395281
- PMCID: PMC8358612
- DOI: 10.3389/fonc.2021.706652
Prognostic Role of Tumor Mutational Burden in Cancer Patients Treated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis
Abstract
Purpose: Immunotherapy is regarded as the most promising treatment for cancer. However, immune checkpoint inhibitors (ICIs) are not effective for all patients. Herein, we conducted a systematic review and meta-analysis to explore whether tumor mutational burden (TMB) can be used as a potential prognostic biomarker for cancer patients treated with ICIs.
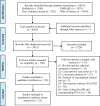
Methods: We systematically retrieved relevant literature published in the PubMed, Embase, Web of Science, and Cochrane databases up to December 28, 2020. All cohort studies and clinical trials that reported hazard ratios (HRs) for overall (OS) and progression-free survival (PFS), as well as the corresponding 95% confidence intervals (CIs) of high and low TMB patients, were included. All statistical analyses were performed using the R software.
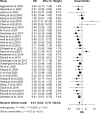
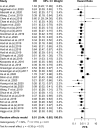
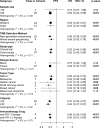
Results: Pooled results from a total of 32 studies with 6,131 participants showed significantly increased OS (HR: 0.61, 95% CI: 0.53-0.71; P <0.01) and PFS (HR: 0.51, 95% CI: 0.44-0.60; P <0.01) for the high TMB group receiving ICIs as compared to the low TMB group. Particularly, results were found to be more significant in studies with larger sample sizes (≥30), Western patients, higher TMB cutoff values (≥20 mut/Mb), anti-PD-1 therapy, and when the sample source was tissue and tumor type was either melanoma, small cell lung cancer, or gastric cancer.
Conclusion: TMB is a promising independent prognostic biomarker for cancer patients receiving ICIs, which could provide a new potential therapeutic strategy for high TMB patients who have failed traditional therapy. Furthermore, consistency in the key aspects of TMB assessment is expected in the future.
Systematic review registration: [https://www.crd.york.ac.uk/PROSPERO], Prospective Register of Systematic Reviews (PROSPERO), identifier: CRD42021229016.
Keywords: cutoff; immune checkpoint inhibitor; overall survival; progression-free survival; tumor mutational burden.
Copyright © 2021 Huang, Chen, Zhang, Liang, Li, Wei, Sun and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Powles T, Durán I, van der Heijden MS, Loriot Y, Vogelzang NJ, De Giorgi U, et al. . Atezolizumab Versus Chemotherapy in Patients With Platinum-Treated Locally Advanced or Metastatic Urothelial Carcinoma (Imvigor211): A Multicentre, Open-Label, Phase 3 Randomised Controlled Trial. Lancet (2018) 391(10122):748–57. 10.1016/s0140-6736(17)33297-x - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

