Perennial Ryegrass Contains Gluten-Like Proteins That Could Contaminate Cereal Crops
- PMID: 34395501
- PMCID: PMC8355629
- DOI: 10.3389/fnut.2021.708122
Perennial Ryegrass Contains Gluten-Like Proteins That Could Contaminate Cereal Crops
Abstract
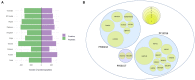
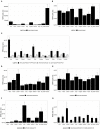
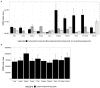
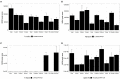
Background: To ensure safe consumption of gluten-free products, there is a need to understand all sources of unintentional contamination with gluten in the food chain. In this study, ryegrass (Lolium perenne), a common weed infesting cereal crop, is analysed as a potential source of gluten-like peptide contamination. Materials and Methods: Ten ryegrass cultivars were analysed using shotgun proteomics for the presence of proteins from the prolamin superfamily. A relative quantitative assay was developed to detect ryegrass gluten-like peptides in comparison with those found in 10 common wheat cultivars. Results: A total of 19 protein accessions were found across 10 cultivars of ryegrass for the protein families of PF00234-Tryp_alpha_amyl, PF13016-Gliadin, and PF03157-Glutenin_HMW. Protein and peptide homology searches revealed that gliadin-like peptides were similar to avenin and gamma-gliadin peptides. A total of 20 peptides, characteristic of prolamin superfamily proteins, were selected for liquid chromatography mass spectrometry (LC-MS) with multiple reaction monitoring (MRM). Only two of the monitored peptides were detected with high abundance in wheat, and all others were detected in ryegrass. Glutenin and alpha-amylase/trypsin inhibitor peptides were reported for the first time in ryegrass and were noted to be conserved across the Poaceae family. Conclusion: A suite of gluten-like peptides were identified using proteomics that showed consistent abundance across ryegrass cultivars but were not detected in wheat cultivars. These peptides will be useful for differentiating wheat gluten contamination from ryegrass gluten contamination.
Keywords: LC-MS/MS; cereal; gluten; proteomics; ryegrass; wheat; wild grass.
Copyright © 2021 Escobar-Correas, Broadbent, Andraszek, Stockwell, Howitt, Juhász and Colgrave.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources

