Meropenem Versus Piperacillin-Tazobactam for Definitive Treatment of Bloodstream Infections Caused by AmpC β-Lactamase-Producing Enterobacter spp, Citrobacter freundii, Morganella morganii, Providencia spp, or Serratia marcescens: A Pilot Multicenter Randomized Controlled Trial (MERINO-2)
- PMID: 34395716
- PMCID: PMC8361238
- DOI: 10.1093/ofid/ofab387
Meropenem Versus Piperacillin-Tazobactam for Definitive Treatment of Bloodstream Infections Caused by AmpC β-Lactamase-Producing Enterobacter spp, Citrobacter freundii, Morganella morganii, Providencia spp, or Serratia marcescens: A Pilot Multicenter Randomized Controlled Trial (MERINO-2)
Abstract
Background: Carbapenems are recommended treatment for serious infections caused by AmpC-producing gram-negative bacteria but can select for carbapenem resistance. Piperacillin-tazobactam may be a suitable alternative.
Methods: We enrolled adult patients with bloodstream infection due to chromosomal AmpC producers in a multicenter randomized controlled trial. Patients were assigned 1:1 to receive piperacillin-tazobactam 4.5 g every 6 hours or meropenem 1 g every 8 hours. The primary efficacy outcome was a composite of death, clinical failure, microbiological failure, and microbiological relapse at 30 days.
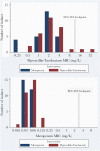
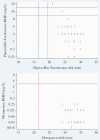
Results: Seventy-two patients underwent randomization and were included in the primary analysis population. Eleven of 38 patients (29%) randomized to piperacillin-tazobactam met the primary outcome compared with 7 of 34 patients (21%) in the meropenem group (risk difference, 8% [95% confidence interval {CI}, -12% to 28%]). Effects were consistent in an analysis of the per-protocol population. Within the subcomponents of the primary outcome, 5 of 38 (13%) experienced microbiological failure in the piperacillin-tazobactam group compared to 0 of 34 patients (0%) in the meropenem group (risk difference, 13% [95% CI, 2% to 24%]). In contrast, 0% vs 9% of microbiological relapses were seen in the piperacillin-tazobactam and meropenem arms, respectively. Susceptibility to piperacillin-tazobactam and meropenem using broth microdilution was found in 96.5% and 100% of isolates, respectively. The most common AmpC β-lactamase genes identified were bla CMY-2, bla DHA-17, bla CMH-3, and bla ACT-17. No ESBL, OXA, or other carbapenemase genes were identified.
Conclusions: Among patients with bloodstream infection due to AmpC producers, piperacillin-tazobactam may lead to more microbiological failures, although fewer microbiological relapses were seen.
Clinical trials registration: NCT02437045.
Keywords: Enterobacterales; ampC β-lactamase; carbapenem; clinical trial; piperacillin-tazobactam.
© The Author(s) 2021. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Figures

References
-
- Meini S, Tascini C, Cei M, et al. . AmpC β-lactamase-producing Enterobacterales: what a clinician should know. Infection 2019; 47:363–75. - PubMed
-
- Mizrahi A, Delerue T, Morel H, et al. ; Saint-Joseph/Avicenna Study Group. Infections caused by naturally AmpC-producing Enterobacteriaceae: can we use third-generation cephalosporins? A narrative review. Int J Antimicrob Agents 2020; 55:105834. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

