Optimization of time domain diffuse correlation spectroscopy parameters for measuring brain blood flow
- PMID: 34395719
- PMCID: PMC8358828
- DOI: 10.1117/1.NPh.8.3.035005
Optimization of time domain diffuse correlation spectroscopy parameters for measuring brain blood flow
Abstract
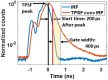
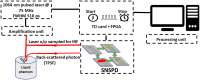
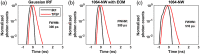
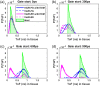
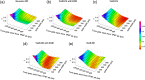
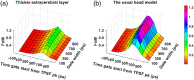
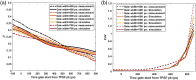
Significance: Time domain diffuse correlation spectroscopy (TD-DCS) can offer increased sensitivity to cerebral hemodynamics and reduced contamination from extracerebral layers by differentiating photons based on their travel time in tissue. We have developed rigorous simulation and evaluation procedures to determine the optimal time gate parameters for monitoring cerebral perfusion considering instrumentation characteristics and realistic measurement noise. Aim: We simulate TD-DCS cerebral perfusion monitoring performance for different instrument response functions (IRFs) in the presence of realistic experimental noise and evaluate metrics of sensitivity to brain blood flow, signal-to-noise ratio (SNR), and ability to reject the influence of extracerebral blood flow across a variety of time gates to determine optimal operating parameters. Approach: Light propagation was modeled on an MRI-derived human head geometry using Monte Carlo simulations for 765- and 1064-nm excitation wavelengths. We use a virtual probe with a source-detector separation of 1 cm placed in the pre-frontal region. Performance metrics described above were evaluated to determine optimal time gate(s) for different IRFs. Validation of simulation noise estimates was done with experiments conducted on an intralipid-based liquid phantom. Results: We find that TD-DCS performance strongly depends on the system IRF. Among Gaussian pulse shapes, pulse length appears to offer the best performance, at wide gates (500 ps and larger) with start times 400 and 600 ps after the peak of the TPSF at 765 and 1064 nm, respectively, for a 1-s integration time at photon detection rates seen experimentally (600 kcps at 765 nm and 4 Mcps at 1064 nm). Conclusions: Our work shows that optimal time gates satisfy competing requirements for sufficient sensitivity and sufficient SNR. The achievable performance is further impacted by system IRF with quasi-Gaussian pulse obtained using electro-optic laser shaping providing the best results.
Keywords: Monte Carlo simulation; cerebral blood flow measurement; instrument response function; optimization; time domain diffuse correlation spectroscopy.
© 2021 The Authors.
Figures