COVID-19: a Disease with a Potpourri of Histopathologic Findings-a Literature Review and Comparison to the Closely Related SARS and MERS
- PMID: 34396046
- PMCID: PMC8354305
- DOI: 10.1007/s42399-021-01029-5
COVID-19: a Disease with a Potpourri of Histopathologic Findings-a Literature Review and Comparison to the Closely Related SARS and MERS
Abstract
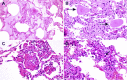
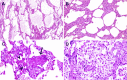
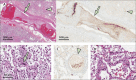
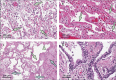
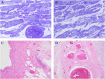
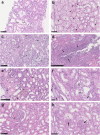
Since the coronavirus disease 2019 (COVID-19) pandemic has hit the entire world, there is ample knowledge regarding its clinical course and prognostic biomarkers. Still, the pathophysiology of COVID-19 is poorly understood. Since the first guidelines published in February 2020 for autopsy of both confirmed and suspected COVID-19 cases, there has been an increasing number of autopsies and literature reporting histopathological findings. However, our knowledge about the immunological response of various organ systems to the virus, as well as response patterns, is inadequate but is essential to understand and initiate timely and targeted antiviral, anti-inflammatory, or anticoagulative therapy. Although severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is primarily considered a respiratory virus, current evidence shows that it causes life-threatening complications in almost all organ systems including the heart, brain, kidney, spleen, liver, and eyes. Hence, in this article, we reviewed the published case reports and case series in order to increase our understanding of COVID-19 pathophysiology. The main histopathological findings of the lungs include diffuse alveolar damage with activated type II pneumocytes, fibroblasts, protein-rich exudate, and hyaline membranes. Other significant histopathological findings include cardiomegaly, right ventricular dilation, splenic pulp atrophy, kidneys with severe podocytopathy, and collapsing glomerulopathy, and the brain showed hypoxic changes in the cerebellum and cerebrum. Furthermore, in this review, we also explained different pathological findings of SARS-CoV and MERS and compared them to SARS-CoV-2. This comprehensive review will improve our understanding of COVID-19 pathophysiology and various disease stages, hence promoting the application of targeted therapy.
Keywords: COVID-19; Coronavirus; Diffuse alveolar damage; Pathology; SARS-CoV-2.
© The Author(s), under exclusive licence to Springer Nature Switzerland AG 2021.
Conflict of interest statement
Conflict of InterestThe authors declare no competing interests.
Figures




Similar articles
-
Evidence of Severe Acute Respiratory Syndrome Coronavirus 2 Replication and Tropism in the Lungs, Airways, and Vascular Endothelium of Patients With Fatal Coronavirus Disease 2019: An Autopsy Case Series.J Infect Dis. 2021 Mar 3;223(5):752-764. doi: 10.1093/infdis/jiab039. J Infect Dis. 2021. PMID: 33502471 Free PMC article.
-
Comparison of RNA In Situ Hybridization and Immunohistochemistry Techniques for the Detection and Localization of SARS-CoV-2 in Human Tissues.Am J Surg Pathol. 2021 Jan;45(1):14-24. doi: 10.1097/PAS.0000000000001563. Am J Surg Pathol. 2021. PMID: 32826529
-
Antemortem vs Postmortem Histopathologic and Ultrastructural Findings in Paired Transbronchial Biopsy Specimens and Lung Autopsy Samples From Three Patients With Confirmed SARS-CoV-2.Am J Clin Pathol. 2022 Jan 6;157(1):54-63. doi: 10.1093/ajcp/aqab087. Am J Clin Pathol. 2022. PMID: 34463314 Free PMC article.
-
Human and novel coronavirus infections in children: a review.Paediatr Int Child Health. 2021 Feb;41(1):36-55. doi: 10.1080/20469047.2020.1781356. Epub 2020 Jun 25. Paediatr Int Child Health. 2021. PMID: 32584199 Review.
-
Histopathological findings and clinicopathologic correlation in COVID-19: a systematic review.Mod Pathol. 2021 Sep;34(9):1614-1633. doi: 10.1038/s41379-021-00814-w. Epub 2021 May 24. Mod Pathol. 2021. PMID: 34031537 Free PMC article.
Cited by
-
Histopathologic findings on indication renal allograft biopsies after recovery from acute COVID-19.Clin Transplant. 2021 Dec;35(12):e14486. doi: 10.1111/ctr.14486. Epub 2021 Oct 1. Clin Transplant. 2021. PMID: 34532893 Free PMC article.
-
Risk of New-Onset Liver Injuries Due to COVID-19 in Preexisting Hepatic Conditions-Review of the Literature.Medicina (Kaunas). 2022 Dec 28;59(1):62. doi: 10.3390/medicina59010062. Medicina (Kaunas). 2022. PMID: 36676691 Free PMC article. Review.
-
Atypical follicular hyperplasia with light chain-restricted germinal centers after COVID-19 booster: a diagnostic pitfall.Virchows Arch. 2023 May;482(5):905-910. doi: 10.1007/s00428-022-03400-w. Epub 2022 Sep 13. Virchows Arch. 2023. PMID: 36098816 Free PMC article.
-
2 Years into the Pandemic: What Did We Learn About the COVID-19 and Cerebellum?Cerebellum. 2022 Feb;21(1):19-22. doi: 10.1007/s12311-021-01351-7. Cerebellum. 2022. PMID: 35088299 Free PMC article.
References
-
- COVID-19 CORONAVIRUS PANDEMIC. (2020) Worldometer. https://www.worldometers.info/coronavirus/#countries. Accessed January 27 2021
-
- United States COVID-19 Cases and Deaths by State. (2020) Centers for Disease Control and Prevention (CDC). https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed January27, 2021
-
- Patel U, Malik P, Usman MS, Mehta D, Sharma A, Malik FA, Khan N, Siddiqi TJ, Ahmed J, Patel A, Sacks H (2020) Age-adjusted risk factors associated with mortality and mechanical ventilation utilization amongst COVID-19 hospitalizations-a systematic review and meta-analysis. SN Compr Clin Med:1-10. doi:10.1007/s42399-020-00476-w, 2, 1740, 1749 - PMC - PubMed
-
- Edler C, Schröder AS, Aepfelbacher M, Fitzek A, Heinemann A, Heinrich F, Klein A, Langenwalder F, Lütgehetmann M, Meißner K, Püschel K, Schädler J, Steurer S, Mushumba H, Sperhake JP. Dying with SARS-CoV-2 infection-an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int J Legal Med. 2020;134(4):1275–1284. doi: 10.1007/s00414-020-02317-w. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
