Effect of anakinra on mortality in patients with COVID-19: a systematic review and patient-level meta-analysis
- PMID: 34396156
- PMCID: PMC8352496
- DOI: 10.1016/S2665-9913(21)00216-2
Effect of anakinra on mortality in patients with COVID-19: a systematic review and patient-level meta-analysis
Abstract
Background: Anakinra might improve the prognosis of patients with moderate to severe COVID-19 (ie, patients requiring oxygen supplementation but not yet receiving organ support). We aimed to assess the effect of anakinra treatment on mortality in patients admitted to hospital with COVID-19.
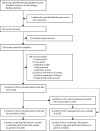
Methods: For this systematic review and individual patient-level meta-analysis, a systematic literature search was done on Dec 28, 2020, in Medline (PubMed), Cochrane, medRxiv, bioRxiv, and the ClinicalTrials.gov databases for randomised trials, comparative studies, and observational studies of patients admitted to hospital with COVID-19, comparing administration of anakinra with standard of care, or placebo, or both. The search was repeated on Jan 22, 2021. Individual patient-level data were requested from investigators and corresponding authors of eligible studies; if individual patient-level data were not available, published data were extracted from the original reports. The primary endpoint was mortality after 28 days and the secondary endpoint was safety (eg, the risk of secondary infections). This study is registered on PROSPERO (CRD42020221491).
Findings: 209 articles were identified, of which 178 full-text articles fulfilled screening criteria and were assessed. Aggregate data on 1185 patients from nine studies were analysed, and individual patient-level data on 895 patients were provided from six of these studies. Eight studies were observational and one was a randomised controlled trial. Most studies used historical controls. In the individual patient-level meta-analysis, after adjusting for age, comorbidities, baseline ratio of the arterial partial oxygen pressure divided by the fraction of inspired oxygen (PaO2/FiO2), C-reactive protein (CRP) concentrations, and lymphopenia, mortality was significantly lower in patients treated with anakinra (38 [11%] of 342) than in those receiving standard of care with or without placebo (137 [25%] of 553; adjusted odds ratio [OR] 0·32 [95% CI 0·20-0·51]). The mortality benefit was similar across subgroups regardless of comorbidities (ie, diabetes), ferritin concentrations, or the baseline PaO2/FiO2. In a subgroup analysis, anakinra was more effective in lowering mortality in patients with CRP concentrations higher than 100 mg/L (OR 0·28 [95% CI 0·17-0·47]). Anakinra showed a significant survival benefit when given without dexamethasone (OR 0·23 [95% CI 0·12-0·43]), but not with dexamethasone co-administration (0·72 [95% CI 0·37-1·41]). Anakinra was not associated with a significantly increased risk of secondary infections when compared with standard of care (OR 1·35 [95% CI 0·59-3·10]).
Interpretation: Anakinra could be a safe, anti-inflammatory treatment option to reduce the mortality risk in patients admitted to hospital with moderate to severe COVID-19 pneumonia, especially in the presence of signs of hyperinflammation such as CRP concentrations higher than 100 mg/L.
Funding: Sobi.
© 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
EJG-B has received honoraria from AbbVie USA, Abbott CH, Biotest Germany, Brahms, InflaRx, MSD Greece, XBiotech, and Angelini Italy; independent educational grants from AbbVie, Abbott CH, Astellas Pharma Europe, AxisShield, bioMérieux, InflaRx, the Medicines Company and XBiotech; and funding from the FrameWork 7 program HemoSpec (granted to the National and Kapodistrian University of Athens), the Horizon2020 Marie-Curie Project European Sepsis Academy (granted to the National and Kapodistrian University of Athens), and the Horizon 2020 European Grant ImmunoSep (granted to the Hellenic Institute for the Study of Sepsis). MG has received speakers' fees and unrestricted grants from Novartis and Sobi. PP, MKo, and EKo are funded by a COVID-19 grant paid to the Radboud University Medical Center (Radboudumc). JE-O is a co-founder, shareholder, and CSO of ViroGates, Denmark, and named inventor on patents on suPAR owned by Copenhagen University Hospital Hvidovre, Denmark. GK has received from ROCHE-CHUGAI Research Grants (<€20 000), fees from Sobi France for scientific presentations (<€4000) and participated in a SOBI Advisory Board on COVID (unpaid) and in an OLATEC Monitoring Board (unpaid). GCa has received speakers' and consulting fees from Novartis and Sobi. LD has received grants (paid to LD's institution outside the current work) from AbbVie, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Kiniksa, Merk Sharp & Dohme, Mundipharma Pharmaceuticals, Novartis, Pfizer, Roche, Sanofi Genzyme, and Sobi; and consulting fees from AbbVie, Amgen, Biogen, Bristol-Myers Squibb, Celltrion, Galapagos, GlaxoSmithKline, Kiniksa, Novartis, Pfizer, Roche, Sanofi-Genzyme, and Sobi. GH reports consultancy fees from Bristol-Myers Squibb, Lilly, Novartis; speakers' fees from AbbVie, Bristol-Myers-Squibb, Celgene, Lilly, Novartis, Pfizer, Roche, Sanofi-Aventis; support for attending meetings from Bristol-Myers-Squibb, Fresenius-Kabi, Janssen-Cilag, Lilly, Mylan, Roche, UCB; and participation on advisory boards for Bristol-Myers-Squibb and Lilly. FV has received (via the Institut de Recherche pour le Développement) Horizon 2020-EDCTP-European Grants: PANDORA and ITAIL-COVID. All other authors declare no competing interests.
Figures


Comment in
-
Safety and efficacy of interleukin-1 antagonists in hospitalized patients with COVID-19.Eur J Intern Med. 2023 Mar;109:117-119. doi: 10.1016/j.ejim.2022.11.014. Epub 2022 Nov 16. Eur J Intern Med. 2023. PMID: 36462963 Free PMC article. No abstract available.
References
-
- WHO Coronavirus disease (COVID-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019?gclid=Cj...
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

