Prokineticin Receptor-1 Signaling Inhibits Dose- and Time-Dependent Anthracycline-Induced Cardiovascular Toxicity Via Myocardial and Vascular Protection
- PMID: 34396166
- PMCID: PMC8352030
- DOI: 10.1016/j.jaccao.2019.06.003
Prokineticin Receptor-1 Signaling Inhibits Dose- and Time-Dependent Anthracycline-Induced Cardiovascular Toxicity Via Myocardial and Vascular Protection
Abstract
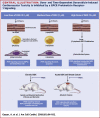
Objectives: This study investigated how different concentrations of doxorubicin (DOX) can affect the function of cardiac cells. This study also examined whether activation of prokineticin receptor (PKR)-1 by a nonpeptide agonist, IS20, prevents DOX-induced cardiovascular toxicity in mouse models.
Background: High prevalence of heart failure during and following cancer treatments remains a subject of intense research and therapeutic interest.
Methods: This study used cultured cardiomyocytes, endothelial cells (ECs), and epicardium-derived progenitor cells (EDPCs) for in vitro assays, tumor-bearing models, and acute and chronic toxicity mouse models for in vivo assays.
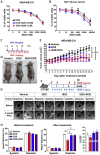
Results: Brief exposure to cardiomyocytes with high-dose DOX increased the accumulation of reactive oxygen species (ROS) by inhibiting a detoxification mechanism via stabilization of cytoplasmic nuclear factor, erythroid 2. Prolonged exposure to medium-dose DOX induced apoptosis in cardiomyocytes, ECs, and EDPCs. However, low-dose DOX promoted functional defects without inducing apoptosis in EDPCs and ECs. IS20 alleviated detrimental effects of DOX in cardiac cells by activating the serin threonin protein kinase B (Akt) or mitogen-activated protein kinase pathways. Genetic or pharmacological inactivation of PKR1 subdues these effects of IS20. In a chronic mouse model of DOX cardiotoxicity, IS20 normalized an elevated serum marker of cardiotoxicity and vascular and EDPC deficits, attenuated apoptosis and fibrosis, and improved the survival rate and cardiac function. IS20 did not interfere with the cytotoxicity or antitumor effects of DOX in breast cancer lines or in a mouse model of breast cancer, but it did attenuate the decreases in left ventricular diastolic volume induced by acute DOX treatment.
Conclusions: This study identified the molecular and cellular signature of dose-dependent, DOX-mediated cardiotoxicity and provided evidence that PKR-1 is a promising target to combat cardiotoxicity of cancer treatments.
Keywords: DMSO, dimethyl sulfoxide; EC, endothelial cell; EDPC, epicardium-derived progenitor cell; EF, ejection fraction; FS, fractional shortening; GPCR, G-protein–coupled receptor; HAEC, human aortic endothelial cell; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; MAPK, mitogen-activated protein kinase; NRF2, nuclear factor, erythroid 2 like 2 (also known as NFE2L2); PECAM, platelet and endothelial cell adhesion molecule; PKR1, prokineticin receptor-1 (also known as PROKR1); PKR1-KO, prokineticin receptor 1 knockout mice; PROK1, prokineticin 1; PROK2, prokineticin 2; TUNEL, terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labeling; breast cancer; doxorubicin; endothelial dysfunction; epicardial progenitor cells; heart failure.
© 2019 The Authors.
Figures







References
-
- van Dalen E.C., van der Pal H.J., Caron H.N., Kremer L.C. Different dosage schedules for reducing cardiotoxicity in cancer patients receiving anthracycline chemotherapy. Cochrane Database Syst Rev. 2006:CD005008. - PubMed
-
- Pugazhendhi A., Edison T., Velmurugan B.K., Jacob J.A., Karuppusamy I. Toxicity of doxorubicin (Dox) to different experimental organ systems. Life Sci. 2018;200:26–30. - PubMed
-
- Wang S.W., Konorev E.A., Kotamraju S., Joseph J., Kalivendi S., Kalyanaraman B. Doxorubicin induces apoptosis in normal and tumor cells via distinctly different mechanisms - intermediacy of H2O2- and p53-dependent pathways. J Biol Chem. 2004;279:25535–25543. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

