Immune Checkpoint Inhibitor-Related Adverse Cardiovascular Events in Patients With Lung Cancer
- PMID: 34396181
- PMCID: PMC8352266
- DOI: 10.1016/j.jaccao.2019.11.013
Immune Checkpoint Inhibitor-Related Adverse Cardiovascular Events in Patients With Lung Cancer
Abstract
Objectives: The purpose of this study was to evaluate whether immune checkpoint inhibitors (ICIs) are associated with an increased risk of major adverse cardiovascular events (MACE) compared with non-ICI therapies in patients with lung cancer.
Background: ICIs activate the host immune system to target cancer cells. Though uncommon, cardiovascular immune-related adverse events can be life-threatening.
Methods: A retrospective single-institution cohort study of 252 patients with pathologically confirmed lung cancer who received ICI or non-ICI therapy was analyzed. The primary endpoint was MACE, defined as a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, and hospitalization for heart failure.
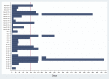
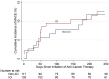
Results: During a median follow-up of 6 months, MACE occurred in 13.3% of ICI-treated patients, with a median time to event of 51 days, compared with 10.3% and 64 days in non-ICI patients. ICIs were not associated with MACE (hazard ratio [HR]: 1.18; 95% confidence interval [CI]: 0.57 to 2.43; p = 0.66) in a univariable Fine-Gray regression analysis incorporating noncardiovascular death as a competing risk. Multivariable regression analyses determined that patients treated with ICIs with elevated serum troponin I >0.01 ng/ml (HR: 7.27; 95% CI: 2.72 to 19.43; p < 0.001) and B-type natriuretic peptide (BNP) >100 pg/ml (HR: 2.65; 95% CI: 1.01 to 6.92; p = 0.047) had an increased risk of MACE. Patients pre-treated or receiving combined immunotherapy with ICIs and vascular endothelial growth factor inhibitors (VEGFIs) or tyrosine kinase inhibitors (TKIs) had an increased risk of MACE (HR: 2.15; 95% CI: 1.05 to 4.37; p = 0.04).
Conclusions: ICIs were not independently associated with an increased risk of MACE in patients with lung cancer, although power is an important limitation in these analyses. ICI-associated cardiotoxicity was associated with elevations in serum troponin and BNP, and combined immunotherapy with VEGFIs or TKIs. Future studies are needed to further define the role of cardiac biomarkers as a monitoring strategy with ICI therapy.
Keywords: BNP; BNP, B-type natriuretic peptide; CI, confidence interval; HR, hazard ratio; ICI, immune checkpoint inhibitor; IQR, interquartile range; LVEF, left ventricular ejection fraction; MACE; MACE, major adverse cardiovascular events; PD, programmed cell death protein; PD-L1, programmed cell death-ligand 1; TKI, tyrosine kinase inhibitor; TnI, troponin I; VEGFI, vascular endothelial growth factor inhibitor; cardiotoxicity; immune checkpoint inhibitors; lung cancer; troponin.
© 2019 The Authors.
Figures

References
-
- Herbst R.S., Baas P., Kim D.W. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. - PubMed
-
- Horn L., Mansfield A.S., Szczesna A. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379:2220–2229. - PubMed
-
- Johnson D.B., Chandra S., Sosman J.A. Immune checkpoint inhibitor toxicity in 2018. JAMA. 2018;320:1702–1703. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

