Oncotherapeutic Protein Kinase Inhibitors Associated With Pro-Arrhythmic Liability
- PMID: 34396309
- PMCID: PMC8352262
- DOI: 10.1016/j.jaccao.2021.01.009
Oncotherapeutic Protein Kinase Inhibitors Associated With Pro-Arrhythmic Liability
Abstract
Background: Ibrutinib is a protein kinase inhibitor that has been widely successful in treating multiple common variations of B-cell cancers. However, an unfortunate side effect of ibrutinib is that it predisposes patients to development of atrial fibrillation.
Objectives: The purpose of this study was to assess other commonly prescribed protein kinase inhibitors for similar pro-arrhythmic liability.
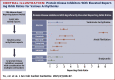
Methods: This study comprehensively evaluated data from the U.S. Food and Drug Administration adverse events reporting system and determined the reporting of cardiac arrhythmia attributed to kinase inhibitor therapy using a multivariable logistic regression model. We evaluated 3,663,300 case reports containing 23,067 cases of atrial fibrillation and 66,262 cases of cardiac arrhythmia. In total, 32 protein kinase inhibitors were evaluated, almost all of which are oncotherapeutics.
Results: Seven protein kinase inhibitors were associated with a significant increase in the odds of atrial fibrillation (ibrutinib, ponatinib, nilotinib, ribociclib, trametinib, osimertinib, and idelalisib). Assessment of broader pro-arrhythmic toxicity suggested a ventricular-specific liability for nilotinib and a bradyarrhythmia risk with alectinib and crizotinib.
Conclusions: Compounds that result in the inhibition of a number of protein kinases are associated with an increased risk of cardiac rhythm disturbances. The mechanisms driving the arrhythmogenic effects remain to be discovered, but this study presents an important step in identifying and prioritizing the study of these protein kinase signaling pathways.
Keywords: AF, atrial fibrillation; FAERS, FDA Adverse Event Reporting System; FDA, U.S. Food and Drug Administration; HLGT, high level group terms; HLT, high level terms; MedDRA, Medical Dictionary for Regulatory Activities; PKI, protein kinase inhibitor; PT, preferred term; ROR, reporting odds ratio; SOC, system organ classes; alectinib; cardiac arrhythmia; cardiotoxicity; crizotinib; ibrutinib; idelalisib; nilotinib; osimertinib; pharmacovigilance; ponatinib; protein kinase inhibitor; ribociclib; risk models; trametinib U.S. Food and Drug Administration Adverse Event Reporting System.
© 2021 The Authors.
Conflict of interest statement
Supported by the Novo Nordisk Foundation grant NNF20OC0059767 to Dr. Lundby. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures




References
-
- Alexandre J., Salem J.-E., Moslehi J. Identification of anticancer drugs associated with atrial fibrillation—analysis of the WHO pharmacovigilance database. Eur Heart J Cardiovasc Pharmacother. 2020:pvaa037. - PubMed
-
- Salem J.E., Manouchehri A., Bretagne M. Cardiovascular toxicities associated with ibrutinib. J Am Coll Cardiol. 2019;74:1667–1678. - PubMed
LinkOut - more resources
Full Text Sources

