Effectiveness of inactivated SARS-CoV-2 vaccines against the Delta variant infection in Guangzhou: a test-negative case-control real-world study
- PMID: 34396940
- PMCID: PMC8425710
- DOI: 10.1080/22221751.2021.1969291
Effectiveness of inactivated SARS-CoV-2 vaccines against the Delta variant infection in Guangzhou: a test-negative case-control real-world study
Abstract
The effectiveness of inactivated SARS-CoV-2 vaccines against the Delta variant, which has been associated with greater transmissibility and virulence, remains unclear. We conducted a test-negative case-control study to explore the vaccine effectiveness (VE) in real-world settings. We recruited participants aged 18-59 years who consisted of SARS-CoV-2 test-positive cases (n = 74) and test-negative controls (n = 292) during the outbreak of the Delta variant in May 2021 in Guangzhou city, China. Vaccination status was compared to estimate The VE of SARS-CoV-2 inactivated vaccines. A single dose of inactivated SARS-CoV-2 vaccine yielded the VE of only 13.8%. After adjusting for age and sex, the overall VE for two-dose vaccination was 59.0% (95% confidence interval: 16.0% to 81.6%) against coronavirus disease 2019 (COVID-19) and 70.2% (95% confidence interval: 29.6-89.3%) against moderate COVID-19 and 100% against severe COVID-19 which might be overestimated due to the small sample size. The VE of two-dose vaccination against COVID-19 reached 72.5% among participants aged 40-59 years, and was higher in females than in males against COVID-19 and moderate diseases. While single dose vaccination was not sufficiently protective, the two-dose dosing scheme of the inactivated vaccines was effective against the Delta variant infection in real-world settings, with the estimated efficacy exceeding the World Health Organization minimal threshold of 50%.
Keywords: Coronavirus disease 2019; Delta variant; SARS-CoV-2; efficacy of vaccination; vaccine.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
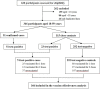
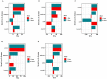
References
-
- Palacios R, Patino EG, de Oliveira Piorelli R, et al. Double-blind, randomized, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of treating healthcare professionals with the adsorbed COVID-19 (inactivated) vaccine manufactured by sinovac – PROFISCOV: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020 Oct 15;21(1):853. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
