Cancer-associated mesothelial cells promote ovarian cancer chemoresistance through paracrine osteopontin signaling
- PMID: 34396988
- PMCID: PMC8363279
- DOI: 10.1172/JCI146186
Cancer-associated mesothelial cells promote ovarian cancer chemoresistance through paracrine osteopontin signaling
Abstract
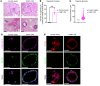
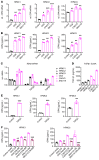
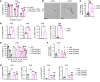
Ovarian cancer is the leading cause of gynecological malignancy-related deaths, due to its widespread intraperitoneal metastases and acquired chemoresistance. Mesothelial cells are an important cellular component of the ovarian cancer microenvironment that promote metastasis. However, their role in chemoresistance is unclear. Here, we investigated whether cancer-associated mesothelial cells promote ovarian cancer chemoresistance and stemness in vitro and in vivo. We found that osteopontin is a key secreted factor that drives mesothelial-mediated ovarian cancer chemoresistance and stemness. Osteopontin is a secreted glycoprotein that is clinically associated with poor prognosis and chemoresistance in ovarian cancer. Mechanistically, ovarian cancer cells induced osteopontin expression and secretion by mesothelial cells through TGF-β signaling. Osteopontin facilitated ovarian cancer cell chemoresistance via the activation of the CD44 receptor, PI3K/AKT signaling, and ABC drug efflux transporter activity. Importantly, therapeutic inhibition of osteopontin markedly improved the efficacy of cisplatin in both human and mouse ovarian tumor xenografts. Collectively, our results highlight mesothelial cells as a key driver of ovarian cancer chemoresistance and suggest that therapeutic targeting of osteopontin may be an effective strategy for enhancing platinum sensitivity in ovarian cancer.
Keywords: Cancer; Cytokines; Oncology; Signal transduction.
Conflict of interest statement
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

