Measurement Properties of Patient-Reported Outcome Measures for Diabetes: Systematic Review
- PMID: 34397387
- PMCID: PMC8398743
- DOI: 10.2196/25002
Measurement Properties of Patient-Reported Outcome Measures for Diabetes: Systematic Review
Abstract
Background: The management of diabetes is complex. There is growing recognition of the use of patient-reported outcome measures (PROMs) as a standardized method of obtaining an outlook on patients' functional status and well-being. However, no systematic reviews have summarized the studies that investigate the measurement properties of diabetes PROMs.
Objective: Our aims were to conduct a systematic review of studies investigating the measurement properties of diabetes PROMs by evaluating the methodological quality and overall level of evidence of these PROMs and to categorize them based on the outcome measures assessed.
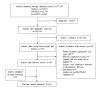
Methods: This study was guided by the PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analysis) guidelines. Relevant articles were retrieved from the Embase, PubMed, and PsychINFO databases. The PROMs were evaluated with the COSMIN (COnsensus-based Standards for the selection of health Measurement Instruments) guidelines.
Results: A total of 363 articles evaluating the measurement properties of PROMs for diabetes in the adult population were identified, of which 238 unique PROMs from 248 studies reported in 209 articles were validated in the type 2 diabetes population. PROMs with at least a moderate level of evidence for ≥5 of 9 measurement properties include the Chinese version of the Personal Diabetes Questionnaire (C-PDQ), Diabetes Self-Management Instrument Short Form (DSMI-20), and Insulin Treatment Appraisal Scale in Hong Kong primary care patients (C-ITAS-HK), of which the C-PDQ has a "sufficient (+)" rating for >4 measurement properties. A total of 43 PROMs meet the COSMIN guidelines for recommendation for use.
Conclusions: This study identified and synthesized evidence for the measurement properties of 238 unique PROMs for patients with type 2 diabetes and categorized the PROMs according to their outcome measures. These findings may assist clinicians and researchers in selecting appropriate high-quality PROMs for clinical practice and research.
Trial registration: PROSPERO International Prospective Register of Systematic Reviews CRD42020180978; https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020180978.
Keywords: PROMs; diabetes; level of evidence; measurement properties; methodological quality; patient reported outcome; patient-reported outcome measures; systematic review.
©Priscilla Jia Ling Wee, Yu Heng Kwan, Dionne Hui Fang Loh, Jie Kie Phang, Troy H Puar, Truls Østbye, Julian Thumboo, Sungwon Yoon, Lian Leng Low. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 13.08.2021.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
Comment in
-
Challenges in Measuring What Matters to Patients With Diabetes. Comment on "Measurement Properties of Patient-Reported Outcome Measures for Diabetes: Systematic Review".J Med Internet Res. 2022 Mar 31;24(3):e36876. doi: 10.2196/36876. J Med Internet Res. 2022. PMID: 35357322 Free PMC article. No abstract available.
-
Authors' Reply to: Challenges in Measuring What Matters to Patients With Diabetes. Comment on "Measurement Properties of Patient-Reported Outcome Measures for Diabetes: Systematic Review".J Med Internet Res. 2022 Mar 31;24(3):e37957. doi: 10.2196/37957. J Med Internet Res. 2022. PMID: 35357327 Free PMC article. No abstract available.
References
-
- IDF Diabetes Atlas, 9th edition. International Diabetes Federation. 2019. [2020-08-01]. https://www.diabetesatlas.org.
-
- American Diabetes Association 1. Promoting health and reducing disparities in populations. Diabetes Care. 2016 Dec 15;40(Supplement 1):S6–S10. doi: 10.2337/dc17-s004. http://care.diabetesjournals.org/lookup/doi/10.2337/dc17-S004 - DOI - PubMed
-
- Skovlund SE, Lichtenberg T, Hessler D, Ejskjaer N. Can the routine use of patient-reported outcome measures improve the delivery of person-centered diabetes care? A review of recent developments and a case study. Curr Diab Rep. 2019 Aug 16;19(9):1–18. doi: 10.1007/s11892-019-1190-x. http://link.springer.com/10.1007/s11892-019-1190-x - DOI - PubMed
-
- Weldring T, Smith SM. Article Commentary: Patient-Reported Outcomes (PROs) and Patient-Reported Outcome Measures (PROMs) Health Serv Ins. 2013 Aug 04;6:HSI.S11093–68. doi: 10.4137/hsi.s11093. http://journals.sagepub.com/doi/10.4137/HSI.S11093 - DOI - PMC - PubMed
-
- Boyer JG, Earp JAL. The development of an instrument for assessing the quality of life of people with diabetes. Med Care. 1997 May;35(5):440–453. doi: 10.1097/00005650-199705000-00003. http://journals.lww.com/00005650-199705000-00003 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical