Switching to Bictegravir/Emtricitabine/Tenofovir Alafenamide in Black Americans With HIV-1: A Randomized Phase 3b, Multicenter, Open-Label Study
- PMID: 34397746
- PMCID: PMC8357046
- DOI: 10.1097/QAI.0000000000002731
Switching to Bictegravir/Emtricitabine/Tenofovir Alafenamide in Black Americans With HIV-1: A Randomized Phase 3b, Multicenter, Open-Label Study
Abstract
Background: With the highest rates of HIV/AIDS in the United States, Black Americans are still underrepresented in HIV medical research.
Setting: BRAAVE (NCT03631732) is a randomized, phase 3b, multicenter, open-label US study.
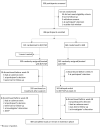
Methods: Adults identifying as Black or African American and virologically suppressed on 2 nucleoside reverse transcriptase inhibitors (NRTIs) plus third agent were randomized (2:1) to switch to open-label bictegravir/emtricitabine/tenofovir alafenamide (B/F/TAF) once daily or stay on baseline regimen (SBR) for 24 weeks, after which SBR had delayed switch to B/F/TAF. Resistance to non-NRTIs, protease inhibitors, and/or NRTIs was permitted; integrase strand transfer inhibitor resistance was exclusionary. Primary endpoint was proportion of participants with HIV-1 RNA ≥50 copies/mL at week 24 (snapshot algorithm; noninferiority margin of 6%).
Results: Of 558 screened, 495 were randomized/treated (B/F/TAF n = 330; SBR n = 165). Overall, 32% were ciswomen, 2% transwomen, and 10% had an M184V/I mutation. At week 24, 0.6% on B/F/TAF vs 1.8% on SBR had HIV-1 RNA ≥50 copies/mL (difference -1.2%; 95% confidence interval -4.8% to 0.9%), demonstrating noninferiority of B/F/TAF vs SBR. Proportions with HIV-1 RNA <50 copies/mL at week 24 were 96% B/F/TAF and 95% SBR and remained high at week 48. No participant had treatment-emergent resistance to study drug. Treatments were well tolerated. Study drug-related adverse events, mostly grade 1, occurred in 10% of participants on B/F/TAF through week 48 and led to discontinuation in 9 participants through week 48.
Conclusions: For Black Americans with HIV, switching to B/F/TAF was noninferior to continuing a variety of regimens, including those with pre-existing NRTI mutations.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
P.K. reports grants from Gilead Sciences, Inc. (Gilead), GlaxoSmithKline (GSK), Merck, and Theratechnologies, has received personal fees for participating in advisory boards for GSK, Merck, and Theratechnologies, and is a shareholder of stock from Gilead, GSK, Johnson & Johnson, Merck, and Pfizer. M.S. has received research grants and support awarded to his institution from Gilead, Merck, and ViiV Healthcare. A.K.W. has received research grants from Gilead, Janssen, Pfizer, and GSK and has served on advisory boards for Gilead and Janssen. I.B. has received speakers' bureau honoraria from Gilead, ViiV, and Janssen. C.O.H. has received grant support from the National Institutes of Health (K23HL116209) and has served as consultant to Gilead Sciences. M.N.R. has served on the speakers' bureau for Gilead, Janssen, AbbVie, and Allergan and has consulted for Gilead, ViiV, and Merck. C.M. reports receiving research grants from Gilead, Janssen, Merck, and ViiV and speaker's bureau income from Gilead and Merck. C.B., K.A., S.E.C., D.M.B., and H.M. are employees of Gilead and shareholders of Gilead stock. The remaining authors have no conflicts of interest to disclose.
Figures

References
-
- Centers for Disease Control and Prevention. HIV Surveillance Report, 2018. Vol 31. 2020. Available at: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed June 26, 2020.
-
- Centers for Disease Control and Prevention. Estimated HIV Incidence and Prevalence in the United States, 2014–2018. HIV Surveillance Supplemental Report 2020. Vol 25. 2020. Available at: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed June 26, 2020.
-
- Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care, Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington (DC): National Academies Press (US); 2003. - PubMed

