Treatment Escalation vs Immediate Initiation of Highly Effective Treatment for Patients With Relapsing-Remitting Multiple Sclerosis: Data From 2 Different National Strategies
- PMID: 34398221
- PMCID: PMC8369379
- DOI: 10.1001/jamaneurol.2021.2738
Treatment Escalation vs Immediate Initiation of Highly Effective Treatment for Patients With Relapsing-Remitting Multiple Sclerosis: Data From 2 Different National Strategies
Abstract
Importance: Treatment strategies for relapsing-remitting multiple sclerosis (RRMS) vary markedly between Denmark and Sweden. The difference in the association of these national strategies with clinical outcomes is unknown.
Objective: To investigate the association of national differences in disease-modifying treatment (DMT) strategies for RRMS with disability outcomes.
Design, setting, and participants: This cohort study used data on 4861 patients from the Danish and Swedish national multiple sclerosis (MS) registries from the date of index DMT initiation (between January 1, 2013, and December 31, 2016) until the last recorded visit at time of data extraction (October 2, 2019).
Exposures: All MS-specific DMTs initiated during the observation period were included in the analysis.
Main outcomes and measures: The primary study outcome was time to 24-week confirmed disability worsening. Secondary outcomes were 24-week confirmed disability improvement, milestone Expanded Disability Status Scale scores of 3 and 4, annualized relapse rate, time to first relapse, and treatment switching. Data were analyzed using inverse probability of treatment weighting-based models using a propensity score to weight and correct the comparison for the imbalance of confounders observed at baseline between the 2 countries.
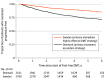
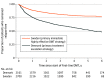
Results: A total of 2700 patients from the Swedish MS registry (1867 women [69.2%]; mean [SD] age, 36.1 [9.5] years) and 2161 patients from the Danish MS registry (1472 women [68.1%]; mean [SD] age, 37.3 [9.4 years]) started a first DMT between 2013 and 2016, were included in the analysis, and were observed for a mean (SD) of 4.1 (1.5) years. A total of 1994 Danish patients (92.3%) initiated a low to moderately effective DMT (teriflunomide, 907 [42.0%]) and 165 (7.6%) initiated a highly effective DMT, whereas a total of 1769 Swedish patients (65.5%) initiated a low to moderately effective DMT (teriflunomide, 64 [2.4%]) and 931 (34.5%) initiated a highly effective DMT. The Swedish treatment strategy was associated with a 29% reduction in the rate of postbaseline 24-week confirmed disability worsening relative to the Danish treatment strategy (hazard ratio, 0.71; 95% CI, 0.57-0.90; P = .004). The Swedish treatment strategy was also associated with a 24% reduction in the rate of reaching an expanded disability status scale score of 3 (hazard ratio, 0.76; 95% CI, 0.60-0.97; P = .03) and a 25% reduction in the rate of reaching an expanded disability status scale score of 4 (hazard ratio, 0.75; 95% CI, 0.61-0.96; P = .01) relative to Danish patients.
Conclusions and relevance: The findings of this study suggest that there is an association between differences in treatment strategies for RRMS and disability outcomes at a national level. Escalation of treatment efficacy was inferior to using more efficacious DMT as initial treatment.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources