Neurofilament light chain and glial fibrillary acidic protein levels in metachromatic leukodystrophy
- PMID: 34398223
- PMCID: PMC8967093
- DOI: 10.1093/brain/awab304
Neurofilament light chain and glial fibrillary acidic protein levels in metachromatic leukodystrophy
Abstract
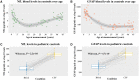
Metachromatic leukodystrophy is a lethal metabolic leukodystrophy, with emerging treatments for early disease stages. Biomarkers to measure disease activity are required for clinical assessment and treatment follow-up. This retrospective study compared neurofilament light chain and glial fibrillary acidic protein (GFAP) levels in CSF (n = 11) and blood (n = 92) samples of 40 patients with metachromatic leukodystrophy (aged 0-42 years) with 38 neurologically healthy children (aged 0-17 years) and 38 healthy adults (aged 18-45 years), and analysed the associations between these levels with clinical phenotype and disease evolution in untreated and transplanted patients. Metachromatic leukodystrophy subtype was determined based on the (expected) age of symptom onset. Disease activity was assessed by measuring gross motor function deterioration and brain MRI. Longitudinal analyses with measurements up to 23 years after diagnosis were performed using linear mixed models. CSF and blood neurofilament light chain and GFAP levels in paediatric controls were negatively associated with age (all P < 0.001). Blood neurofilament light chain level at diagnosis (median, interquartile range; picograms per millilitre) was significantly increased in both presymptomatic (14.7, 10.6-56.7) and symptomatic patients (136, 40.8-445) compared to controls (5.6, 4.5-7.1), and highest among patients with late-infantile (456, 201-854) or early-juvenile metachromatic leukodystrophy (291.0, 104-445) and those ineligible for treatment based on best practice (291, 57.4-472). GFAP level (median, interquartile range; picogram per millilitre) was only increased in symptomatic patients (591, 224-1150) compared to controls (119, 78.2-338) and not significantly associated with treatment eligibility (P = 0.093). Higher blood neurofilament light chain and GFAP levels at diagnosis were associated with rapid disease progression in late-infantile (P = 0.006 and P = 0.051, respectively) and early-juvenile patients (P = 0.048 and P = 0.039, respectively). Finally, blood neurofilament light chain and GFAP levels decreased during follow-up in untreated and transplanted patients but remained elevated compared with controls. Only neurofilament light chain levels were associated with MRI deterioration (P < 0.001). This study indicates that both proteins may be considered as non-invasive biomarkers for clinical phenotype and disease stage at clinical assessment, and that neurofilament light chain might enable neurologists to make better informed treatment decisions. In addition, neurofilament light chain holds promise assessing treatment response. Importantly, both biomarkers require paediatric reference values, given that their levels first decrease before increasing with advancing age.
Keywords: arylsulfatase A; biomarker; glial fibrillary acidic protein; metachromatic leukodystrophy; neurofilament light.
© The Author(s) (2021). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures


References
-
- Von Figura K, Gieselmann V, Jaeken J.. Metachromatic leukodystrophy. In: Scriver CR, Beaudet AL, Sly WS, Valle D, eds. The metabolic and molecular bases of inherited disease. McGraw-Hill; 2001:3695–3724.
-
- Groeschel S, Kehrer C, Engel C, et al. . Metachromatic leukodystrophy: Natural course of cerebral MRI changes in relation to clinical course. J Inherit Metab Dis. 2011;34(5):1095–1102. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

