Turning scientific serendipity into discoveries in breast cancer research and treatment: a tale of PhD students and a 50-year roaming tamoxifen team
- PMID: 34398352
- PMCID: PMC8557169
- DOI: 10.1007/s10549-021-06356-8
Turning scientific serendipity into discoveries in breast cancer research and treatment: a tale of PhD students and a 50-year roaming tamoxifen team
Abstract
Purpose: This retrospective, about a single "mobile" laboratory in six locations on two continents, is intended as a case study in discovery for trainees and junior faculty in the medical sciences. Your knowledge of your topic is necessary to expect the unexpected.
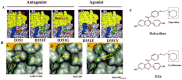
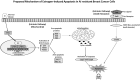
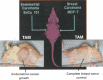
Historical method: In 1972, there was no tamoxifen, only ICI 46, 474, a non-steroidal anti-estrogen with little chance of clinical development. No one would ever be foolish enough to predict that the medicine, 20 years later, would achieve legendary status as the first targeted treatment for breast cancer, and millions of women would benefit from long-term adjuvant tamoxifen therapy. The secret of tamoxifen's success was a translational research strategy proposed in the mid 1970's. This strategy was to treat only patients with estrogen receptor (ER)-positive breast cancer and deploy 5 or more years of adjuvant tamoxifen therapy to prevent recurrence. Additionally, tamoxifen prevented mammary cancer in animals. Could the medicine prevent breast cancer in women?
Results: Tamoxifen and the failed breast cancer drug raloxifene became the first selective estrogen receptor modulators (SERMs): a new drug group, discovered at the University of Wisconsin, Comprehensive Cancer Center. Serendipity can play a fundamental role in discovery, but there must be a rigorous preparation for the investigator to appreciate the possibility of a pending discovery. This article follows the unanticipated discoveries when PhD students "get the wrong answer." The secret of success of my six Tamoxifen Teams was their technical excellence to create models, to decipher mechanisms, that drove the development of new medicines. Discoveries are listed that either changed women's health or allowed an understanding of originally opaque mechanisms of action of potential therapies. These advances in women's health were supported entirely by government-sponsored peer-reviewed funding and major philanthropy from the Lynn Sage Breast Cancer Foundation, the Avon Foundation, and the Susan G. Komen Breast Cancer Foundation. The resulting lives saved or extended, families aided in a time of crisis and the injection of billions of dollars into national economies by drug development, is proof of the value of Federal or philanthropic investment into unencumbered research aimed at saving millions of lives.
Keywords: Estrogen receptor; Raloxifene; Selective estrogen receptor modulators; Tamoxifen; Women’s Health Initiative.
© 2021. The Author(s).
Conflict of interest statement
The author declares that he has no conflicts of interest.
Figures



References
-
- Watson, J, (2010) Avoid Boring People, Vintage Books, a division of Random House New York.
-
- Eig J. The Birth of the Pill. London: Pan Macmillan a division of Macmillan Publishers LTD; 2015.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

