Theranostic applications of optical coherence tomography in neurosurgery?
- PMID: 34398385
- PMCID: PMC8827310
- DOI: 10.1007/s10143-021-01599-x
Theranostic applications of optical coherence tomography in neurosurgery?
Abstract
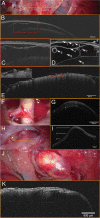
In light of our own experiences, we value the existing literature to critically point out possible "near" future applications of optical coherence tomography (OCT) as an intraoperative neurosurgical guidance tool. "Pub Med", "Cochrane Library", "Crossref Metadata Search", and "IEEE Xplore" databases as well as the search engine "Google Scholar" were screened for "optical coherence tomography + neurosurgery", "optical coherence tomography + intraoperative imaging + neurosurgery", and "microscope integrated optical coherence tomography + neurosurgery". n = 51 articles related to the use of OCT as an imaging technique in the field of neurosurgery or neurosurgical research. n = 7 articles documented the intraoperative use of OCT in patients. n = 4 articles documented the use of microscope-integrated optical coherence tomography as a neurosurgical guidance tool. The Results demonstrate that OCT is the first imaging technique to study microanatomy in vivo. Postoperative analysis of intraoperative scans holds promise to enrich our physiological and pathophysiological understanding of the human brain. No data exists to prove that OCT-guided surgery minimizes perioperative morbidity or extends tumor resection. But results suggest that regular use of microscope-integrated OCT could increase security during certain critical microsurgical steps like, e.g., dural dissection at cavernous sinus, transtentorial approaches, or aneurysm clip placement. Endoscopy integration could aid surgery in regions which are not yet accessible to real-time imaging modalities like the ventricles or hypophysis. Theranostic instruments which combine OCT with laser ablation might gain importance in the emerging field of minimal invasive tumor surgery. OCT depicts vessel wall layers and its pathologies uniquely. Doppler OCT could further visualize blood flow in parallel. These abilities shed light on promising future applications in the field of vascular neurosurgery.
Keywords: Intraoperative imaging; Microscope integration; Neurosurgery; Optical coherence tomography.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests. OptoMedical Technologies GmbH supported our research group with free equipment for iOCT.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

