Determinants of Primaquine and Carboxyprimaquine Exposures in Children and Adults with Plasmodium vivax Malaria
- PMID: 34398667
- PMCID: PMC8522776
- DOI: 10.1128/AAC.01302-21
Determinants of Primaquine and Carboxyprimaquine Exposures in Children and Adults with Plasmodium vivax Malaria
Abstract
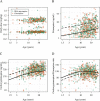
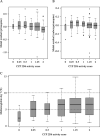
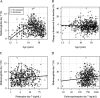
Primaquine is the only widely available drug for radical cure of Plasmodium vivax malaria. There is uncertainty whether the pharmacokinetic properties of primaquine are altered significantly in childhood or not. Patients with uncomplicated P. vivax malaria and with normal glucose-6-phosphate dehydrogenase were randomized to receive either chloroquine (25 mg base/kg of body weight) or dihydroartemisinin-piperaquine (dihydroartemisinin at 7 mg/kg and piperaquine at 55 mg/kg) plus primaquine, given either as 0.5 mg base/kg/day for 14 days or 1 mg/kg/day for 7 days. Predose day 7 venous plasma concentrations of chloroquine, desethylchloroquine, piperaquine, primaquine, and carboxyprimaquine were measured. Methemoglobin levels were measured at frequent intervals. Day 7 primaquine and carboxyprimaquine concentrations were available for 641 patients. After adjustment for the milligram-per-kilogram primaquine daily dose, day of sampling, partner drug, and fever clearance, there was a significant nonlinear relationship between age and trough primaquine and carboxyprimaquine concentrations and daily methemoglobin levels. Compared to adults 30 years of age, children 5 years of age had trough primaquine concentrations that were 0.53 (95% confidence interval [CI], 0.39 to 0.73)-fold lower, trough carboxyprimaquine concentrations that were 0.45 (95% CI, 0.35 to 0.55)-fold lower, and day 7 methemoglobin levels that were 0.87 (95% CI, 0.58 to 1.27)-fold lower. Increasing plasma concentrations of piperaquine and chloroquine and poor metabolizer CYP 2D6 alleles were associated with higher day 7 primaquine and carboxyprimaquine plasma concentrations. Higher blood methemoglobin concentrations were associated with a lower risk of recurrence. Young children have lower primaquine and carboxyprimaquine exposures and lower levels of methemoglobinemia than adults. Young children may need higher weight-adjusted primaquine doses than adults. (This study has been registered at ClinicalTrials.gov under identifier NCT01640574.).
Keywords: Plasmodium vivax; chloroquine; dihydroartemisinin-piperaquine; pharmacokinetics; primaquine; radical cure; relapse.
Figures
References
-
- World Health Organization. 2015. Guidelines for the treatment of malaria, 3rd ed. World Health Organization, Geneva, Switzerland.
-
- Moore BR, Salman S, Benjamin J, Page-Sharp M, Robinson LJ, Waita E, Batty KT, Siba P, Mueller I, Davis TME, Betuela I. 2014. Pharmacokinetic properties of single-dose primaquine in Papua New Guinean children: feasibility of abbreviated high-dose regimens for radical cure of vivax malaria. Antimicrob Agents Chemother 58:432–439. 10.1128/AAC.01437-13. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

