Point-of-Care Antigen Test for SARS-CoV-2 in Asymptomatic College Students
- PMID: 34399086
- PMCID: PMC8462309
- DOI: 10.3201/eid2710.210080
Point-of-Care Antigen Test for SARS-CoV-2 in Asymptomatic College Students
Abstract
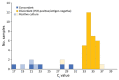
We used the BinaxNOW COVID-19 Ag Card to screen 1,540 asymptomatic college students for severe acute respiratory syndrome coronavirus 2 in a low-prevalence setting. Compared with reverse transcription PCR, BinaxNOW showed 20% overall sensitivity; among participants with culturable virus, sensitivity was 60%. BinaxNOW provides point-of-care screening but misses many infections.
Keywords: 2019 novel coronavirus disease; COVID-19; SARS-CoV-2; adolescents; antigen testing; asymptomatic; colleges; coronavirus disease; coronaviruses; institutions of higher education; respiratory infections; severe acute respiratory syndrome coronavirus 2; universities; viruses; young adults; zoonoses.
Figures
References
-
- US Food and Drug Administration. BinaxNOW COVID-19 Ag Card emergency use authorization. 2020 [cited 2021 Jun 28]. https://www.fda.gov/media/147264/download
-
- Centers for Medicare and Medicaid Services. Updated CLIA SARS-CoV-2 molecular and antigen point of care test enforcement discretion. 2020 [cited 2021 Jun 28]. https://www.cms.gov/files/document/clia-sars-cov-2-point-care-test-enfor...
-
- Georgia Department of Public Health. Covid-19 daily status report, by county (Floyd County, November 16th). 2020. [cited 2021 Feb 28]. https://dph.georgia.gov/covid-19-daily-status-report
-
- US Food and Drug Administration. BinaxNOW COVID-19 Ag Card—instructions for use. 2020. [cited 2021 Jun 28]. https://www.fda.gov/media/141570/download
-
- US Food and Drug Administration. Influenza SARS-CoV-2 (Flu SC2) Multiplex Assay emergency use authorization. 2020. [cited 2021 Jun 28]. https://www.fda.gov/media/139744/download
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous