Lactic acid suppresses MRGPRX2 mediated mast cell responses
- PMID: 34399172
- PMCID: PMC8428143
- DOI: 10.1016/j.cellimm.2021.104422
Lactic acid suppresses MRGPRX2 mediated mast cell responses
Abstract
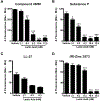
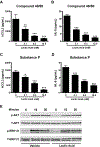
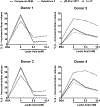
MAS related G-protein coupled receptor X2 (MRGPRX2) is a G-protein coupled receptor (GPCR) expressed in human mast cells that has been implicated to play an important role in causing pseudo-allergic reactions as well as exacerbating inflammation during asthma and other allergic diseases. Lactic acid, a byproduct of glucose metabolism, is abundantly present in inflamed tissues and has been shown to regulate functions of several immune cells. Because the endogenous ligands for MRGPRX2 (substance P and LL-37) are elevated during pathologic conditions, such as cancer and asthma, and given that lactic acid levels are also enhanced in these patients, we explored the role of lactic acid in regulating mast cells response via MRGPRX2 and MrgprB2, the mouse orthologue of the human receptor. We found that lactic acid suppressed both the early (Ca2+ mobilization and degranulation) and late (chemokine/cytokine release) phases of mast cell activation; this data was confirmed in LAD2, human skin and mouse peritoneal mast cells. In LAD2 cells, the reduction in degranulation and chemokine/cytokine production mediated by lactic acid was dependent on pH. In agreement with our in vitro studies, lactic acid also reduced passive systemic anaphylaxis to compound 48/80 (a known MRGPRX2/MrgprB2 ligand) and skin inflammation in a mouse model of rosacea that is dependent on MrgprB2 expression on skin mast cells. Our data thus suggest that lactic acid may serve to inhibit mast cell-mediated inflammation during asthma and reduce immune response during cancer by affecting mast cell activation through MRGPRX2.
Keywords: Lactic acid; MAS-related G-protein coupled receptor-X2 (MRGPRX2); Mast cells; MrgprB2; Pseudo-allergic reactions.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

