Exercise-induced late preconditioning in mice is triggered by eNOS-dependent generation of nitric oxide and activation of PKCε and is mediated by increased iNOS activity
- PMID: 34400167
- PMCID: PMC8453107
- DOI: 10.1016/j.ijcard.2021.08.021
Exercise-induced late preconditioning in mice is triggered by eNOS-dependent generation of nitric oxide and activation of PKCε and is mediated by increased iNOS activity
Abstract
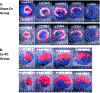
The purpose of this study was to assess whether short-term, mild exercise induces protection against myocardial infarction and, if so, what role the eNOS-PKCε-iNOS axis plays. Mice were subjected to 2 bouts/day of treadmill exercise (60 min at 15 m/min) for 2 consecutive days. At 24 h after the last bout of exercise, mice were subjected to a 30-min coronary artery occlusion and 24 h of reperfusion. In the exercise group (group III, wild-type mice), infarct size (25.5 ± 8.8% of risk region) was significantly (P < 0.05) reduced compared with the control groups (sham exercise, group II [63.4 ± 7.8%] and acute myocardial infarction, group I [58.6 ± 7.0%]). This effect was abolished by pretreatment with the NOS inhibitor L-NA (group VI, 56.1 ± 16.2%) and the PKC inhibitor chelerythrine (group VIII, 57.9 ± 12.5%). Moreover, the late PC effect of exercise was completely abrogated in eNOS-/- mice (group XIII, 61.0 ± 11.2%). The myocardial phosphorylated eNOS at Ser-1177 was significantly increased at 30 min after treadmill training (exercise group) compared with sham-exercised hearts. PKCε translocation was significantly increased at 30 min after exercise in WT mice but not in eNOS-/- mice. At 24 h after exercise, iNOS protein was upregulated compared with sham-exercised hearts. The protection of late PC was abrogated in iNOS-/- mice (group XVI, 56.4 ± 12.9%) and in wildtype mice given the selective iNOS inhibitor 1400 W prior to ischemia (group X 62.0 ± 8.8% of risk region). We conclude that 1) even short, mild exercise induces a delayed PC effect that affords powerful protection against infarction; 2) this cardioprotective effect is dependent on activation of eNOS, eNOS-derived NO generation, and subsequent PKCε activation during PC; 3) the translocation of PKCε is dependent on eNOS; 4) the protection 24 h later is dependent on iNOS activity. Thus, eNOS is the trigger and iNOS the mediator of PC induced by mild exercise.
Keywords: Exercise; Ischemia/reperfusion injury; NOS, nitric oxide synthase; Preconditioning; Protein kinase C.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest
The authors declare no competing interests.
Figures


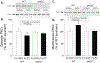



Comment in
-
Nitric oxide as a fragile switch between cardioprotection and cardiac injury.Int J Cardiol. 2021 Nov 15;343:102-103. doi: 10.1016/j.ijcard.2021.09.001. Epub 2021 Sep 6. Int J Cardiol. 2021. PMID: 34499973 No abstract available.
-
LncRNA CPhar mediates exercise-induced cardioprotection by promoting eNOS phosphorylation at Ser1177 via DDX17/PI3K/Akt pathway after MI/RI.Int J Cardiol. 2022 Mar 1;350:16. doi: 10.1016/j.ijcard.2021.12.040. Epub 2021 Dec 24. Int J Cardiol. 2022. PMID: 34954278 No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

