Preclinical Risk Evaluation of Normal Tissue Injury With Novel Radiosensitizers
- PMID: 34400266
- PMCID: PMC8764622
- DOI: 10.1016/j.ijrobp.2021.08.003
Preclinical Risk Evaluation of Normal Tissue Injury With Novel Radiosensitizers
Abstract
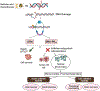
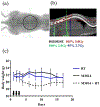
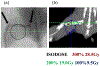
Genotoxic damage induced by radiation triggers a highly coordinated DNA damage response, and molecular inhibitors of key nodes within this complex response network can profoundly enhance the antitumor efficacy of radiation. This is especially true for drugs targeting the catalytic subunit of DNA-dependent protein kinase, which is a core component of the nonhomologous end-joining DNA repair pathway, and ataxia telangiectasia mutated, which coordinates cell cycle arrest, apoptosis, and DNA repair functionalities after radiation exposure. Unlike the more modest in vitro radiosensitizing effects seen with classic sensitizing agents such as cisplatin, 5-fluorouracil, or taxanes, DNA-dependent protein kinase or ataxia telangiectasia mutated inhibitors provide much more robust sensitizing effects in vitro, as might be anticipated from targeting these key DNA repair modulators. However, patients with homozygous inactivating mutations of ataxia telangiectasia mutated or mice with homozygous defects in DNA-dependent protein kinase (severe combined immunodeficiency) have profoundly enhanced acute normal tissue radiation reactions. Therefore, there is significant potential that the combination of small molecule inhibitors of these kinases with radiation could cause similar dose-limiting acute normal tissue toxicities. Similarly, although less understood, inhibition of these DNA repair response pathways could markedly increase the risk of late radiation toxicities. Because these potent radiosensitizers could be highly useful to improve local control of otherwise radiation-resistant tumors, understanding the potential for elevated risks of radiation injury is essential for optimizing therapeutic ratio and developing safe and informative clinical trials. In this review, we will discuss 2 straightforward models to assess the potential for enhanced mucosal toxicity in the oral cavity and small intestine established in our laboratories. We also will discuss similar strategies for evaluating potential drug-radiation interactions with regard to increased risks of debilitating late effects.
Copyright © 2021 Elsevier Inc. All rights reserved.
Figures




References
-
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006;24:4833–4839. - PubMed
-
- Shiloh Y, Ziv Y. The ATM protein kinase: Regulating the cellular response to genotoxic stress, and more. Nat Rev Mol Cell Biol 2013;14:197–210. - PubMed
-
- Zenke FT, Zimmermann A, Sirrenberg C, et al. Pharmacological inhibitor of DNA-pk, m3814, potentiates radiotherapy and regresses human tumors in mouse models. Mol Cancer Ther 2020;19:1091–1101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

