Transposable elements and their KZFP controllers are drivers of transcriptional innovation in the developing human brain
- PMID: 34400477
- PMCID: PMC8415367
- DOI: 10.1101/gr.275133.120
Transposable elements and their KZFP controllers are drivers of transcriptional innovation in the developing human brain
Abstract
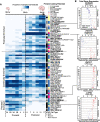
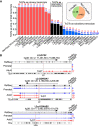
Transposable elements (TEs) account for more than 50% of the human genome and many have been co-opted throughout evolution to provide regulatory functions for gene expression networks. Several lines of evidence suggest that these networks are fine-tuned by the largest family of TE controllers, the KRAB-containing zinc finger proteins (KZFPs). One tissue permissive for TE transcriptional activation (termed "transposcription") is the adult human brain, however comprehensive studies on the extent of this process and its potential contribution to human brain development are lacking. To elucidate the spatiotemporal transposcriptome of the developing human brain, we have analyzed two independent RNA-seq data sets encompassing 16 brain regions from eight weeks postconception into adulthood. We reveal a distinct KZFP:TE transcriptional profile defining the late prenatal to early postnatal transition, and the spatiotemporal and cell type-specific activation of TE-derived alternative promoters driving the expression of neurogenesis-associated genes. Long-read sequencing confirmed these TE-driven isoforms as significant contributors to neurogenic transcripts. We also show experimentally that a co-opted antisense L2 element drives temporal protein relocalization away from the endoplasmic reticulum, suggestive of novel TE dependent protein function in primate evolution. This work highlights the widespread dynamic nature of the spatiotemporal KZFP:TE transcriptome and its importance throughout TE mediated genome innovation and neurotypical human brain development. To facilitate interactive exploration of these spatiotemporal gene and TE expression dynamics, we provide the "Brain TExplorer" web application freely accessible for the community.
© 2021 Playfoot et al.; Published by Cold Spring Harbor Laboratory Press.
Figures




References
-
- Adam SA, Schnell O, Pöschl J, Eigenbrod S, Kretzschmar HA, Tonn J-C, Schüller U. 2012. ALDH1A1 is a marker of astrocytic differentiation during brain development and correlates with better survival in glioblastoma patients. Brain Pathol 22: 788–797. 10.1111/j.1750-3639.2012.00592.x - DOI - PMC - PubMed
-
- Attig J, Young GR, Hosie L, Perkins D, Encheva-Yokoya V, Stoye JP, Snijders AP, Ternette N, Kassiotis G. 2019. LTR retroelement expansion of the human cancer transcriptome and immunopeptidome revealed by de novo transcript assembly. Genome Res 29: 1578–1590. 10.1101/gr.248922.119 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
