Dorsal Root Ganglia Macrophages Maintain Osteoarthritis Pain
- PMID: 34400519
- PMCID: PMC8482866
- DOI: 10.1523/JNEUROSCI.1787-20.2021
Dorsal Root Ganglia Macrophages Maintain Osteoarthritis Pain
Erratum in
-
Erratum: Raoof et al., "Dorsal Root Ganglia Macrophages Maintain Osteoarthritis Pain".J Neurosci. 2022 Feb 9;42(6):1166. doi: 10.1523/JNEUROSCI.2445-21.2021. Epub 2022 Jan 27. J Neurosci. 2022. PMID: 35086908 Free PMC article. No abstract available.
Abstract
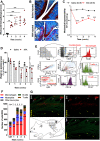
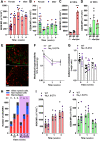
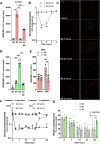
Pain is the major debilitating symptom of osteoarthritis (OA), which is difficult to treat. In OA patients joint tissue damage only poorly associates with pain, indicating other mechanisms contribute to OA pain. Immune cells regulate the sensory system, but little is known about the involvement of immune cells in OA pain. Here, we report that macrophages accumulate in the dorsal root ganglia (DRG) distant from the site of injury in two rodent models of OA. DRG macrophages acquired an M1-like phenotype, and depletion of DRG macrophages resolved OA pain in male and female mice. Sensory neurons innervating the damaged knee joint shape DRG macrophages into an M1-like phenotype. Persisting OA pain, accumulation of DRG macrophages, and programming of DRG macrophages into an M1-like phenotype were independent of Nav1.8 nociceptors. Inhibition of M1-like macrophages in the DRG by intrathecal injection of an IL4-IL10 fusion protein or M2-like macrophages resolved persistent OA pain. In conclusion, these findings reveal a crucial role for macrophages in maintaining OA pain independent of the joint damage and suggest a new direction to treat OA pain.SIGNIFICANCE STATEMENT In OA patients pain poorly correlates with joint tissue changes indicating mechanisms other than only tissue damage that cause pain in OA. We identified that DRG containing the somata of sensory neurons innervating the damaged knee are infiltrated with macrophages that are shaped into an M1-like phenotype by sensory neurons. We show that these DRG macrophages actively maintain OA pain remotely and independent of joint damage. The phenotype of these macrophages is crucial for a pain-promoting role. Targeting the phenotype of DRG macrophages with either M2-like macrophages or a cytokine fusion protein that skews macrophages into an M2-like phenotype resolves OA pain. Our work reveals a mechanism that contributes to the maintenance of OA pain distant from the affected knee joint and suggests that dorsal root ganglia macrophages are a target to treat osteoarthritis chronic pain.
Keywords: chronic pain; macrophage; osteoarthritis; sensory neuron.
Copyright © 2021 the authors.
Figures



References
-
- Arendt-Nielsen L, Simonsen O, Laursen MB, Roos EM, Rathleff MS, Rasmussen S, Skou ST (2018) Pain and sensitization after total knee replacement or nonsurgical treatment in patients with knee osteoarthritis: identifying potential predictors of outcome at 12 months. Eur J Pain 22:1088–1102. 10.1002/ejp.1193 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
