The acute phase protein lactoferrin is a key feature of Alzheimer's disease and predictor of Aβ burden through induction of APP amyloidogenic processing
- PMID: 34400772
- PMCID: PMC8758478
- DOI: 10.1038/s41380-021-01248-1
The acute phase protein lactoferrin is a key feature of Alzheimer's disease and predictor of Aβ burden through induction of APP amyloidogenic processing
Abstract
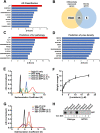
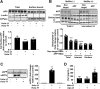
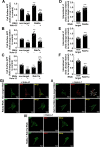
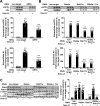
Amyloidogenic processing of the amyloid precursor protein (APP) forms the amyloid-β peptide (Aβ) component of pathognomonic extracellular plaques of AD. Additional early cortical changes in AD include neuroinflammation and elevated iron levels. Activation of the innate immune system in the brain is a neuroprotective response to infection; however, persistent neuroinflammation is linked to AD neuropathology by uncertain mechanisms. Non-parametric machine learning analysis on transcriptomic data from a large neuropathologically characterised patient cohort revealed the acute phase protein lactoferrin (Lf) as the key predictor of amyloid pathology. In vitro studies showed that an interaction between APP and the iron-bound form of Lf secreted from activated microglia diverted neuronal APP endocytosis from the canonical clathrin-dependent pathway to one requiring ADP ribosylation factor 6 trafficking. By rerouting APP recycling to the Rab11-positive compartment for amyloidogenic processing, Lf dramatically increased neuronal Aβ production. Lf emerges as a novel pharmacological target for AD that not only modulates APP processing but provides a link between Aβ production, neuroinflammation and iron dysregulation.
© 2021. The Author(s).
Conflict of interest statement
The authors have no known financial and personal relationships that may inappropriately influence their work. BG is a director of Pacific Analytics PTY LTD and SMRTR PTY LTD, Australia; a founding member of the International Cerebral Palsy Genetics Consortium and a member of the Australian Genomics Health Alliance. AIB is a shareholder in Prana Biotechnology Ltd, Cogstate Ltd, Brighton Biotech LLC, Grunbiotics Pty Ltd, Eucalyptus Pty Ltd and Mesoblast Ltd. He is a paid consultant for, and has a profit share interest in, Collaborative Medicinal Development Pty Ltd.
Figures


References
-
- Milward EA, Papadopoulos R, Fuller SJ, Moir RD, Small D, Beyreuther K, et al. The amyloid protein precursor of Alzheimer’s disease is a mediator of the effects of nerve growth factor on neurite outgrowth. Neuron. 1992;9:129–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

