Immune suppressive checkpoint interactions in the tumour microenvironment of primary liver cancers
- PMID: 34400801
- PMCID: PMC8727557
- DOI: 10.1038/s41416-021-01453-3
Immune suppressive checkpoint interactions in the tumour microenvironment of primary liver cancers
Abstract
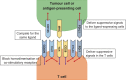
Liver cancer is one of the most prevalent cancers, and the third most common cause of cancer-related mortality worldwide. The therapeutic options for the main types of primary liver cancer-hepatocellular carcinoma (HCC) and cholangiocarcinoma (CCA)-are very limited. HCC and CCA are immunogenic cancers, but effective immune-mediated tumour control is prevented by their immunosuppressive tumour microenvironment. Despite the critical involvement of key co-inhibitory immune checkpoint interactions in immunosuppression in liver cancer, only a minority of patients with HCC respond to monotherapy using approved checkpoint inhibitor antibodies. To develop effective (combinatorial) therapeutic immune checkpoint strategies for liver cancer, in-depth knowledge of the different mechanisms that contribute to intratumoral immunosuppression is needed. Here, we review the co-inhibitory pathways that are known to suppress intratumoral T cells in HCC and CCA. We provide a detailed description of insights from preclinical studies in cellular crosstalk within the tumour microenvironment that results in interactions between co-inhibitory receptors on different T-cell subsets and their ligands on other cell types, including tumour cells. We suggest alternative immune checkpoints as promising targets, and draw attention to the possibility of combined targeting of co-inhibitory and co-stimulatory pathways to abrogate immunosuppression.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

