A heat-shock response regulated by the PfAP2-HS transcription factor protects human malaria parasites from febrile temperatures
- PMID: 34400833
- PMCID: PMC8390444
- DOI: 10.1038/s41564-021-00940-w
A heat-shock response regulated by the PfAP2-HS transcription factor protects human malaria parasites from febrile temperatures
Abstract
Periodic fever is a characteristic clinical feature of human malaria, but how parasites survive febrile episodes is not known. Although the genomes of Plasmodium species encode a full set of chaperones, they lack the conserved eukaryotic transcription factor HSF1, which activates the expression of chaperones following heat shock. Here, we show that PfAP2-HS, a transcription factor in the ApiAP2 family, regulates the protective heat-shock response in Plasmodium falciparum. PfAP2-HS activates the transcription of hsp70-1 and hsp90 at elevated temperatures. The main binding site of PfAP2-HS in the entire genome coincides with a tandem G-box DNA motif in the hsp70-1 promoter. Engineered parasites lacking PfAP2-HS have reduced heat-shock survival and severe growth defects at 37 °C but not at 35 °C. Parasites lacking PfAP2-HS also have increased sensitivity to imbalances in protein homeostasis (proteostasis) produced by artemisinin, the frontline antimalarial drug, or the proteasome inhibitor epoxomicin. We propose that PfAP2-HS contributes to the maintenance of proteostasis under basal conditions and upregulates specific chaperone-encoding genes at febrile temperatures to protect the parasite against protein damage.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Figures








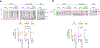



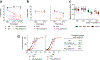

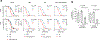
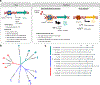
Comment in
-
Malaria parasite beats the heat.Nat Microbiol. 2021 Sep;6(9):1105-1107. doi: 10.1038/s41564-021-00953-5. Nat Microbiol. 2021. PMID: 34400834 No abstract available.
References
-
- Richter K, Haslbeck M & Buchner J. The heat shock response: life on the verge of death. Mol Cell 40, 253–266 (2010). - PubMed
-
- Hartl FU, Bracher A & Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature 475, 324–332 (2011). - PubMed
-
- Anckar J & Sistonen L. Regulation of HSF1 function in the heat stress response: implications in aging and disease. Annu Rev Biochem 80, 1089–1115 (2011). - PubMed
References cited only in the Methods section.
-
- Cortés A, Benet A, Cooke BM, Barnwell JW & Reeder JC Ability of Plasmodium falciparum to invade Southeast Asian ovalocytes varies between parasite lines. Blood 104, 2961–2966 (2004). - PubMed
-
- Cortés A. A chimeric Plasmodium falciparum Pfnbp2b/Pfnbp2a gene originated during asexual growth. Int J Parasitol 35, 125–130 (2005). - PubMed
-
- Walliker D. et al.Genetic analysis of the human malaria parasite Plasmodium falciparum. Science 236, 1661–1666 (1987). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

