AML-derived Extracellular Vesicles Confer De Novo Chemoresistance to Leukemic Myeloblast Cells by Promoting Drug Export Genes Expression and ROS Inhibition
- PMID: 34400967
- PMCID: PMC8170774
- DOI: 10.22037/ijpr.2020.113272.14199
AML-derived Extracellular Vesicles Confer De Novo Chemoresistance to Leukemic Myeloblast Cells by Promoting Drug Export Genes Expression and ROS Inhibition
Abstract
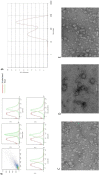
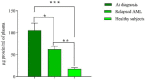
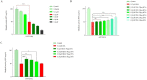
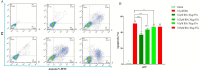
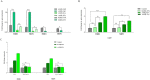
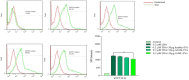
In spite of successful initial remission, chemo-resistance and relapse are still concerning points in acute myeloid leukemia (AML) treatment strategies. Multidrug resistance (MDR) appears to be the major contributor of chemo-resistance, arising in some sub-clones of cancers and could be developed in others. The aim of this study was to investigate the role of extracellular vesicles (EVs) derived from AML patients on the transmission of chemo-resistance phenotype. Ultracentrifugation was employed to isolate EVs from healthy controls, new cases, and relapsed AML patients. The EVs size, morphology, and immunophenotype were determined by dynamic light scattering, TEM, and flow cytometry respectively. Bradford assay was performed to measure the protein content of EVs. MTT assay and flow cytometry analysis were also used to determine the viability index, induction of apoptosis, and ROS generation in U937 cells. The expression level of two efflux pumps was assessed using qRT-PCR analysis. Findings of TEM, DLS, and flow cytometry confirmed that EVs had a desirable shape, size, and surface markers. EVs derived from both new cases and relapsed AML patients significantly reduced idarubicin-induced apoptosis in the U937 cells. The analysis of drug efflux pumps gens revealed that EVs over-express MRD1 and MRP1 in the target cells. These findings suggested a novel role of EVs in mediating the acquired chemo-resistance in AML patients by inducing the expression of the drug efflux pumps; however, further investigations will be required to elucidate other underlying mechanisms of resistance that are mediated by EVs.
Keywords: Acute myeloid leukemia; De novo; Extracellular vesicles; Multidrug resistance; Relapse.
Figures

References
-
- Roug AS, Ommen HB. Clinical use of measurable residual disease in acute myeloid leukemia. Curr. Treat. Options Oncol. . 2019;20:1–12. - PubMed
-
- Rowe JM, Kim HT, Cassileth PA, Lazarus HM, Litzow MR, Wiernik PH, Tallman MS. Adult patients with acute myeloid leukemia who achieve complete remission after 1 or 2 cycles of induction have a similar prognosis: a report on 1980 patients registered to 6 studies conducted by the Eastern Cooperative Oncology Group. Cancer . 2010;116:5012–21. - PMC - PubMed
LinkOut - more resources
Full Text Sources
