Pulmonary delivery of siRNA against acute lung injury/acute respiratory distress syndrome
- PMID: 34401226
- PMCID: PMC8359643
- DOI: 10.1016/j.apsb.2021.08.009
Pulmonary delivery of siRNA against acute lung injury/acute respiratory distress syndrome
Abstract
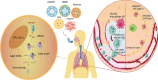
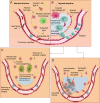
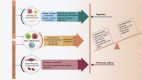
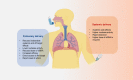
The use of small interfering RNAs (siRNAs) has been under investigation for the treatment of several unmet medical needs, including acute lung injury/acute respiratory distress syndrome (ALI/ARDS) wherein siRNA may be implemented to modify the expression of pro-inflammatory cytokines and chemokines at the mRNA level. The properties such as clear anatomy, accessibility, and relatively low enzyme activity make the lung a good target for local siRNA therapy. However, the translation of siRNA is restricted by the inefficient delivery of siRNA therapeutics to the target cells due to the properties of naked siRNA. Thus, this review will focus on the various delivery systems that can be used and the different barriers that need to be surmounted for the development of stable inhalable siRNA formulations for human use before siRNA therapeutics for ALI/ARDS become available in the clinic.
Keywords: AAV, adeno-associated virus; ALI/ARDS; ALI/ARDS, acute lung injury/acute respiratory distress syndrome; AM, alveolar macrophage; ATI, alveolar cell type I; ATII, alveolar cell type II; AV, adenovirus; Ago-2, argonaute 2; CFDA, China Food and Drug Administration; COPD, chronic obstructive pulmonary disease; CPP, cell-penetrating peptide; CS, cigarette smoke; CXCR4, C–X–C motif chemokine receptor type 4; Cellular uptake; DAMPs, danger-associated molecular patterns; DC-Chol, 3β-(N-(N′,N′-dimethylethylenediamine)-carbamoyl) cholesterol; DDAB, dimethyldioctadecylammonium bromide; DODAP, 1,2-dioleyl-3-dimethylammonium-propane; DODMA, 1,2-dioleyloxy-N,N-dimethyl-3-aminopropane; DOGS, dioctadecyl amido glycin spermine; DOPC, 1,2-dioleoyl-sn-glycero-3-phosphocholine; DOPE, 1,2-dioleoyl-l-α-glycero-3-phosphatidylethanolamine; DOSPA, 2,3-dioleyloxy-N-[2-(sperminecarboxamido)ethyl]-N,N-dimethyl-1-propanaminium; DOTAP, 1,2-dioleoyl-3-trimethylammonium-propane; DOTMA, N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium; DPI, dry powder inhaler; DPPC, 1,2-dipalmitoyl-sn-glycero-3-phosphocholine; Drug delivery; EC, endothelial cell; EPC, egg phosphatidylcholine; EXOs, exosomes; Endosomal escape; EpiC, epithelial cell; FDA, US Food and Drug Administration; HALI, hyperoxic acute lung injury; HMGB1, high-mobility group box 1; HMVEC, human primary microvascular endothelial cell; HNPs, hybrid nanoparticles; Hem-CLP, hemorrhagic shock followed by cecal ligation and puncture septic challenge; ICAM-1, intercellular adhesion molecule-1; IFN, interferons; Inflammatory diseases; LPS, lipopolysaccharides; MEND, multifunctional envelope-type nano device; MIF, macrophage migration inhibitory factor; Myd88, myeloid differentiation primary response 88; N/P ratio, nitrogen /phosphate ratio; NETs, neutrophil extracellular traps; NF-κB, nuclear factor kappa B; NPs, nanoparticles; Nanoparticles; PAI-1, plasminogen activator inhibitor-1; PAMAM, polyamidoamine; PAMPs, pathogen-associated molecular patterns; PD-L1, programmed death ligand-1; PDGFRα, platelet-derived growth factor receptor-α; PEEP, positive end-expiratory pressure; PEG, polyethylene glycol; PEI, polyethyleneimine; PF, pulmonary fibrosis; PFC, perfluorocarbon; PLGA, poly(d,l-lactic-co-glycolic acid); PMs, polymeric micelles; PRR, pattern recognition receptor; PS, pulmonary surfactant; Pulmonary administration; RIP2, receptor-interacting protein 2; RISC, RNA-induced silencing complex; RNAi, RNA interference; ROS, reactive oxygen species; SLN, solid lipid nanoparticle; SNALP, stable nucleic acid lipid particle; TGF-β, transforming growth factor-β; TLR, Toll-like receptor; TNF-α, tumor necrosis factor-α; VALI, ventilator-associated lung injury; VILI, ventilator-induced lung injury; dsDNA, double-stranded DNA; dsRNA, double-stranded RNA; eggPG, l-α-phosphatidylglycerol; mRNA, messenger RNA; miRNA, microRNA; pDNA, plasmid DNA; shRNA, short RNA; siRNA; siRNA, small interfering RNA.
© 2022 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Figures



References
-
- Ostwani W., Shanley T.P. In: Pediatric critical care medicine. Wheeler D., Wong H., Shanley T., editors. Springer-Verlag London Ltd; London: 2014. Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) pp. 101–126.
-
- McNicholas B.A., Rooney G.M., Laffey J.G. Lessons to learn from epidemiologic studies in ARDS. Curr Opin Crit Care. 2018;24:41–48. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

