Long-term follow-up of renal function in patients treated with migalastat for Fabry disease
- PMID: 34401344
- PMCID: PMC8353473
- DOI: 10.1016/j.ymgmr.2021.100786
Long-term follow-up of renal function in patients treated with migalastat for Fabry disease
Erratum in
-
Corrigendum to "Long-term follow-up of renal function in patients treated with migalastat for Fabry disease" [Bichet et al., MGM Reports; 28 (2021) 100786].Mol Genet Metab Rep. 2021 Oct 2;29:100807. doi: 10.1016/j.ymgmr.2021.100807. eCollection 2021 Dec. Mol Genet Metab Rep. 2021. PMID: 34934630 Free PMC article.
Abstract
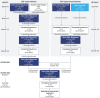
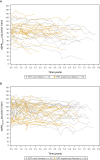
The effect of migalastat on long-term renal outcomes in enzyme replacement therapy (ERT)-naive and ERT-experienced patients with Fabry disease is not well defined. An integrated posthoc analysis of the phase 3 clinical trials and open-label extension studies was conducted to evaluate long-term changes in renal function in patients with Fabry disease and amenable GLA variants who were treated with migalastat for ≥2 years during these studies. The analysis included ERT-naive (n = 36 [23 females]; mean age 45 years; mean baseline estimated glomerular filtration rate (eGFR), 91.4 mL/min/mL/1.73 m2) and ERT-experienced (n = 42 [24 females]; mean age, 50 years; mean baseline eGFR, 89.2 mL/min/1.73m2) patients with amenable variants who received migalastat 123 mg every other day for ≥2 years. The annualized rate of change from baseline to last observation in estimated glomerular filtration rate using the Chronic Kidney Disease Epidemiology Collaboration equation (eGFRCKD-EPI) was calculated by both simple linear regression and a random coefficient model. In ERT-naive patients, mean annualized rates of change from baseline in eGFRCKD-EPI were - 1.6 mL/min/1.73 m2 overall and - 1.8 mL/min/1.73 m2 and - 1.4 mL/min/1.73 m2 in male and female patients, respectively, as estimated by simple linear regression. In ERT-experienced patients, mean annualized rates of change from baseline in eGFRCKD-EPI were - 1.6 mL/min/1.73 m2 overall and - 2.6 mL/min/1.73 m2 and - 0.8 mL/min/1.73 m2 in male and female patients, respectively. Mean annualized rate of change in eGFRCKD-EPI in ERT-naive patients with the classic phenotype (defined by white blood cell alpha galactosidase A [α-Gal A] activity of <3% of normal and multiorgan system involvement) was -1.7 mL/min/1.73 m2. When calculated using the random coefficient model, which adjusted for sex, age, and baseline renal function, the annualized eGFRCKD-EPI change was minimal (mean: -0.1 and 0.1 mL/min/1.73 m2 in ERT-naive and ERT-experienced patients, respectively). In conclusion, patients with Fabry disease and amenable GLA variants receiving long-term migalastat treatment (≤8.6 years) maintained renal function irrespective of treatment status, sex, or phenotype.
Keywords: Chaperone; Classic phenotype; Efficacy; Fabry disease; GLP-HEK, Good Laboratory Practice-validated human embryonic kidney; Gb3, globotriaosylceramide; LVMi, left ventricular mass index; Migalastat; Q1, quartile 1; Q3, quartile 3; RI, renin inhibitor; Renal function; eGFRCKD-EPI, estimated glomerular filtration rate using the Chronic Kidney Disease Epidemiology Collaboration equation; α-Gal A, α-galactosidase A.
© 2021 The Authors. Published by Elsevier Inc.
Conflict of interest statement
D.G.B. served as a consultant and speaker for and received grants from Amicus, Sanofi Genzyme and Otsuka; and served as a board member for Amicus. E.W. received grants and consulting fees from Sanofi Genzyme and received research and travel support from Protalix and Idorsia. D.H. received consultant fees from Amicus, Sanofi Genzyme, Shire-Takeda, Protalix, Idorisia, and Freeline Therapeutics; and served as a speaker for Amicus, Shire-Takeda, and Sanofi Genzyme. R.G. was a paid consultant for JCR Pharmaceuticals, REGENXBIO, and Sigilon; received grants from BioMarin, Novartis, Shire-Takeda, and Ultragenyx; was a paid speaker for Amicus, BioMarin, Chiesi, Janssen, PTC Therapeutics, Sanofi Genzyme, Shire-Takeda, and Ultragenyx; and served as a board member for Amicus, Abeona, Sobi, Sanofi Genzyme, and Shire-Takeda. N.S. was an employee of Amicus at the time of this study. E.K. served as a paid consultant for Amicus. U.F.R. served as a board member and paid consultant for Amicus, Sanofi Genzyme, and Shire-Takeda, and received an unrestricted research grant from Sanofi Genzyme. K.N. has served on advisory boards for Amicus Therapeutics, Sanofi Genzyme, and Shire; has served as a speaker for Amicus Therapeutics; and has received research funding from Sanofi Genzyme and Shire. R.T. and R.S have no conflicts to declare.
Figures
References
-
- Desnick R.J., Ioannou Y., Eng C.M. a-Galactosidase a deficiency: Fabry disease. In: Valle D., editor. The Online Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill Companies, Inc.; 2016.
-
- Alroy J., Sabnis S., Kopp J.B. Renal pathology in Fabry disease. J. Am. Soc. Nephrol. 2002;13(Suppl. 2):S134–S138. - PubMed
-
- Sanchez-Nino M.D., Sanz A.B., Carrasco S., Saleem M.A., Mathieson P.W., Valdivielso J.M. Globotriaosylsphingosine actions on human glomerular podocytes: implications for Fabry nephropathy. Nephrol. Dial. Transplant. 2011;26:1797–1802. - PubMed
-
- Rozenfeld P.A., de Los Angeles Bolla M., Quieto P., Pisani A., Feriozzi S., Neuman P. Pathogenesis of Fabry nephropathy: the pathways leading to fibrosis. Mol. Genet. Metab. 2020;129:132–141. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

