Bacteria - derived short chain fatty acids restore sympathoadrenal responsiveness to hypoglycemia after antibiotic-induced gut microbiota depletion
- PMID: 34401412
- PMCID: PMC8358200
- DOI: 10.1016/j.ynstr.2021.100376
Bacteria - derived short chain fatty acids restore sympathoadrenal responsiveness to hypoglycemia after antibiotic-induced gut microbiota depletion
Abstract
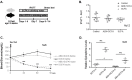
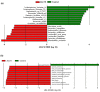
The microbiome co-evolved with their mammalian host over thousands of years. This commensal relationship serves a pivotal role in various metabolic, physiological, and immunological processes. Recently we discovered impaired adrenal catecholamine stress responses in germ-free mice suggesting developmental modification of the reflex arc or absence of an ongoing microbiome signal. To determine whether maturational arrest or an absent bacteria-derived metabolite was the cause, we tested whether depleting gut microbiome in young adult animals could also alter the peripheral stress responses to insulin-induced hypoglycemia. Groups of C57Bl6 male mice were given regular water (control) or a cocktail of non-absorbable broad-spectrum antibiotics (Abx) in the drinking water for two weeks before injection with insulin or saline. Abx mice displayed a profound decrease in microbial diversity and abundance of Bacteroidetes and Firmicutes, plus a markedly enlarged caecum and no detectable by-products of bacterial fermentation (sp. short chain fatty acids, SCFA). Tonic and stress-induced epinephrine levels were attenuated. Recolonization (Abx + R) restored bacterial diversity, but not the sympathoadrenal system responsiveness or caecal acetate, propionate and butyrate levels. In contrast, corticosterone (HPA) and glucagon (parasympathetic) resting values and responses to hypoglycemia remained similar across all conditions. Oral supplementation with SCFA improved epinephrine responses to hypoglycaemia. Whole genome shotgun sequence profiling of fecal samples from control, Abx and Abx + R cohorts identified nine microbes (SCFA producers) absent from both Abx and Abx + R groups. These results implicate gut microbiome depletion plus its attendant reduction in SCFA signalling in adversely affecting the release of epinephrine in response to hypoglycemia. We speculate that regardless of postnatal age, a mutable microbiome messaging system exists throughout life. Unravelling these mechanisms could lead to new therapeutic possibilities through controlled manipulation of the gut microbiota and its ability to alter systemic neurotransmitter responsiveness.
Keywords: Acute hypoglycemia; Epinephrine; Fecal whole genome sequencing; Microbiome; Oral antibiotics; Short chain fatty acids supplement.
© 2021 The Authors.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures



Similar articles
-
Absence of gut microbial colonization attenuates the sympathoadrenal response to hypoglycemic stress in mice: implications for human neonates.Pediatr Res. 2019 Mar;85(4):574-581. doi: 10.1038/s41390-018-0270-y. Epub 2019 Jan 14. Pediatr Res. 2019. PMID: 30675019
-
Oral short-chain fatty acids administration regulates innate anxiety in adult microbiome-depleted mice.Neuropharmacology. 2022 Aug 15;214:109140. doi: 10.1016/j.neuropharm.2022.109140. Epub 2022 May 22. Neuropharmacology. 2022. PMID: 35613660
-
Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain-gut axis alterations.J Physiol. 2018 Oct;596(20):4923-4944. doi: 10.1113/JP276431. Epub 2018 Aug 28. J Physiol. 2018. PMID: 30066368 Free PMC article.
-
Can the gastrointestinal microbiota be modulated by dietary fibre to treat obesity?Ir J Med Sci. 2018 May;187(2):393-402. doi: 10.1007/s11845-017-1686-9. Epub 2017 Oct 16. Ir J Med Sci. 2018. PMID: 29038988 Review.
-
The effects of inulin on gut microbial composition: a systematic review of evidence from human studies.Eur J Clin Microbiol Infect Dis. 2020 Mar;39(3):403-413. doi: 10.1007/s10096-019-03721-w. Epub 2019 Nov 9. Eur J Clin Microbiol Infect Dis. 2020. PMID: 31707507
Cited by
-
What we need to know about the germ-free animal models.AIMS Microbiol. 2024 Feb 6;10(1):107-147. doi: 10.3934/microbiol.2024007. eCollection 2024. AIMS Microbiol. 2024. PMID: 38525038 Free PMC article. Review.
-
Resilience or susceptibility to traumatic stress: Potential influence of the microbiome.Neurobiol Stress. 2022 May 27;19:100461. doi: 10.1016/j.ynstr.2022.100461. eCollection 2022 Jul. Neurobiol Stress. 2022. PMID: 35789769 Free PMC article.
-
Exploring the interaction between the gut microbiota and cyclic adenosine monophosphate-protein kinase A signaling pathway: a potential therapeutic approach for neurodegenerative diseases.Neural Regen Res. 2025 Nov 1;20(11):3095-3112. doi: 10.4103/NRR.NRR-D-24-00607. Epub 2024 Nov 13. Neural Regen Res. 2025. PMID: 39589173 Free PMC article.
-
Washed microbiota transplantation reduces glycemic variability in unstable diabetes.J Diabetes. 2024 Feb;16(2):e13485. doi: 10.1111/1753-0407.13485. Epub 2023 Oct 17. J Diabetes. 2024. PMID: 37846600 Free PMC article.
-
Cold stress initiates catecholaminergic and serotonergic responses in the chicken gut that are associated with functional shifts in the microbiome.Poult Sci. 2024 Mar;103(3):103393. doi: 10.1016/j.psj.2023.103393. Epub 2024 Jan 10. Poult Sci. 2024. PMID: 38320392 Free PMC article.
References
-
- Andrikopoulos S., Blair A.R., Deluca N., Fam B.C., Proietto J. Evaluating the glucose tolerance test in mice. Am. J. Physiol. Endocrinol. Metab. 2008;295:E1323–E1332. - PubMed
-
- Aranyi T., Sarkis C., Berrard S., Sardin K., Siron V., Khalfallah O., Mallet J. Sodium butyrate modifies the stabilizing complexes of tyrosine hydroxylase mRNA. Biochem. Biophys. Res. Commun. 2007;359:15–19. - PubMed
-
- Bercik P., Denou E., Collins J., Jackson W., Lu J., Jury J., Deng Y., Blennerhassett P., Macri J., McCoy K.D. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141:599–609. 609 e591-593. - PubMed
-
- Blacher E., Levy M., Tatirovsky E., Elinav E. Microbiome-modulated metabolites at the interface of host immunity. J. Immunol. 2017;198:572–580. - PubMed
LinkOut - more resources
Full Text Sources

