A mixed-methods study to evaluate a patient-designed tool to reduce harm from cancer-associated thrombosis: The EMPOWER study
- PMID: 34401640
- PMCID: PMC8357625
- DOI: 10.1002/rth2.12545
A mixed-methods study to evaluate a patient-designed tool to reduce harm from cancer-associated thrombosis: The EMPOWER study
Abstract
Introduction: Venous thromboembolism (VTE) is a common and serious complication of systemic anticancer therapies. Delays in presentation increase risk of death or long-term morbidity.
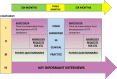
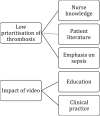
Background: A patient charity developed an information video for patients receiving systemic anticancer therapy including what to do if they developed symptoms of VTE. This was introduced into clinical practice in a regional cancer center and its impact compared with a district general hospital where the video was not used.
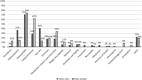
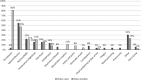
Methods: A mixed-methods approach was used, comprising clinical audit data, patient surveys, and key informant interviews. The time between development of VTE symptoms and seeking medical evaluation was routinely recorded on patients attending a regional cancer-associated thrombosis service with systemic anticancer therapy-provoked VTE. The video was then embedded into clinical practice at the regional cancer center for 3 months. The primary outcome was the difference in time to presentation with VTE symptoms, between patients attending the regional cancer center and the district general hospital (which acted as control). Other outcomes included impact on radiology resources, patient knowledge, and perspectives of chemotherapy nurses.
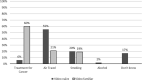
Results: Addition of the video was associated with a lower mean time to presentation from 8.9 to 2.9 days (0.33 hazard ratio; 95% confidence interval, 4.5-7.4; P < .0001). This may reflect greater awareness of VTE, resulting in earlier clinical presentation when they developed attributable symptoms.
Conclusion: The video was associated with reduced delays in diagnosis of systemic anticancer therapy-associated VTE by 6 days, thereby reducing long-term complications.
Keywords: cancer‐associated thrombosis; mixed methods; patient information; qualitative; venous thromboembolism.
© 2021 The Authors. Research and Practice in Thrombosis and Haemostasis published by Wiley Periodicals LLC on behalf of International Society on Thrombosis and Haemostasis (ISTH).
Figures
References
-
- Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer‐associated venous thrombosis. Blood. 2013;122(10):1712‐1723. - PubMed
-
- Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5(3):632‐634. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

